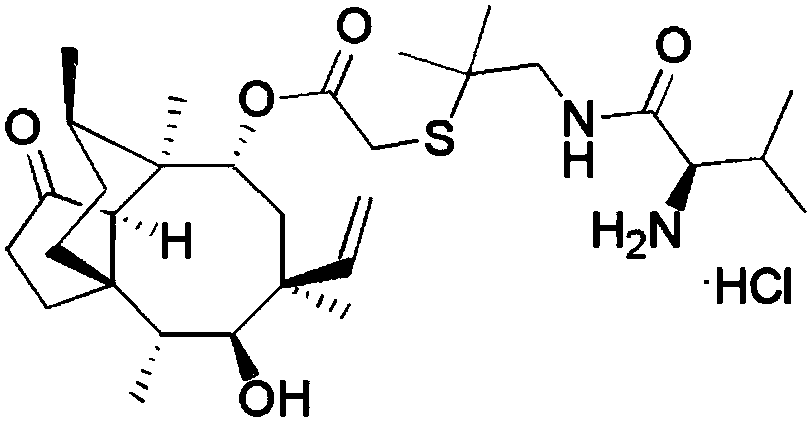
A kind of synthetic method of warnemulin hydrochloride
A technology of vonimulin hydrochloride and synthesis method, which is applied in chemical instruments and methods, preparation of sulfonate esters, preparation of organic compounds, etc. volatilization and other problems, to achieve the effect of promoting the complete reaction, high reaction yield and product purity, and reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
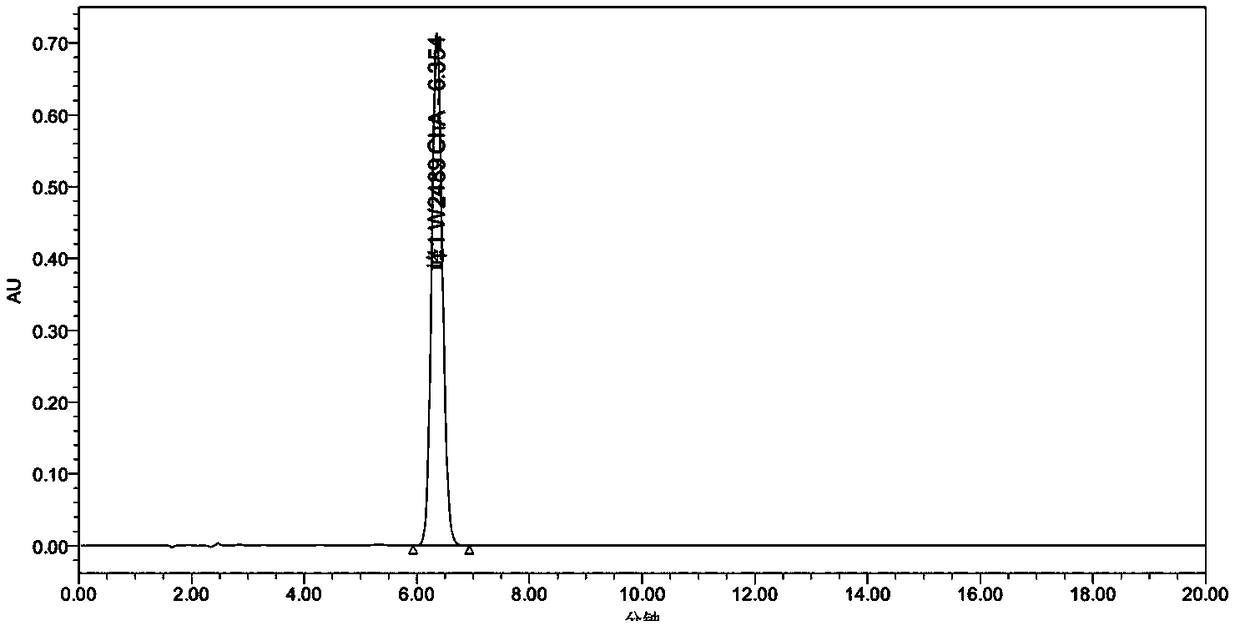
Image
Examples
Embodiment 1
[0058] A kind of synthetic method of warnemulin hydrochloride, specifically comprises the following steps:
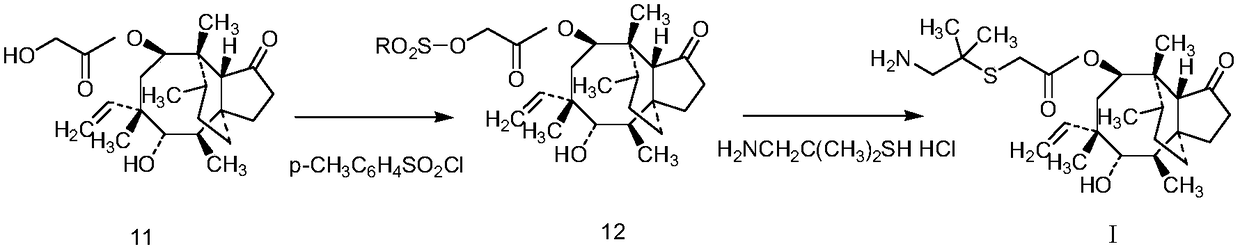
[0059] (1) Synthesis of pleuromutilin with dimethylsemiphotoamine
[0060] In a 500mL four-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a condenser, add compound 11 (75.7g, 0.2mol), 200mL tetrahydrofuran, and 40mL water, and add dropwise a tetrahydrofuran solution of p-toluenesulfonyl chloride ( Dissolve 42g, 0.22mol of p-toluenesulfonyl chloride in 100mL of tetrahydrofuran), add 50mL of 10N NaOH solution after dripping; heat the reaction mixture to reflux, reflux for 1 hour, the reaction solution is yellow and turbid, stop the reaction; filter, filter The cake was dried at 75°C to obtain 96 g of white solid (Compound 12), with a yield of 90.1%.
[0061] In a 500mL three-necked flask equipped with a stirrer, add compound 12 (96g, 0.18mol), a tetrahydrofuran solution of dimethylsemicarcinamide hydrochloride (dissolve 25.5g, 0.18mol dimethyl...
Embodiment 2
[0071] A kind of synthetic method of warnemulin hydrochloride, specifically comprises the following steps:
[0072] (1) Synthesis of pleuromutilin with dimethylsemiphotoamine
[0073] In a 500mL four-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a condenser, add pleuromutilin (133.5g, 0.3mol), 300mL tetrahydrofuran, 60mL water, and add dropwise the tetrahydrofuran of p-toluenesulfonyl chloride under stirring. solution (dissolve 63g, 0.33mol p-toluenesulfonyl chloride in 150mL tetrahydrofuran), then add 75mL 10N NaOH solution; heat the reaction mixture to reflux for one hour, the reaction solution is yellow and turbid, stop the reaction, filter, and filter the cake at 75°C After drying, 145.0 g of white solid (compound 12) was obtained, with a yield of 90.7%.
[0074] In a 500mL three-neck flask equipped with a stirrer, add compound 12 (145.0g, 0.27mol), dimethylsemiphotoamine hydrochloride (38.3g, 0.27mol) and 300mL tetrahydrofuran, and stir at 35°C...
Embodiment 3
[0084] A kind of synthetic method of warnemulin hydrochloride, specifically comprises the following steps:
[0085] (1) Synthesis of pleuromutilin with dimethylsemiphotoamine
[0086] In a 500mL four-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a condenser, add pleuromutilin (75.7g, 0.2mol), 200mL tetrahydrofuran, 40mL water, and add dropwise the tetrahydrofuran of p-toluenesulfonyl chloride under stirring. solution (dissolve 42g, 0.22mol of p-toluenesulfonyl chloride in 100mL of tetrahydrofuran), and then add 50mL of 10N NaOH solution; heat the reaction mixture to reflux for one hour, the reaction solution is yellow and turbid, stop the reaction, filter, and filter the cake at 75°C After drying, 96 g of white solid (compound 12) was obtained, with a yield of 90.1%.
[0087] In a 500mL three-neck flask equipped with a stirrer, add compound 12 (96g, 0.18mol), dimethylsemicarpine hydrochloride (25.5g, 0.18mol) and 200mL tetrahydrofuran, and add ben...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com