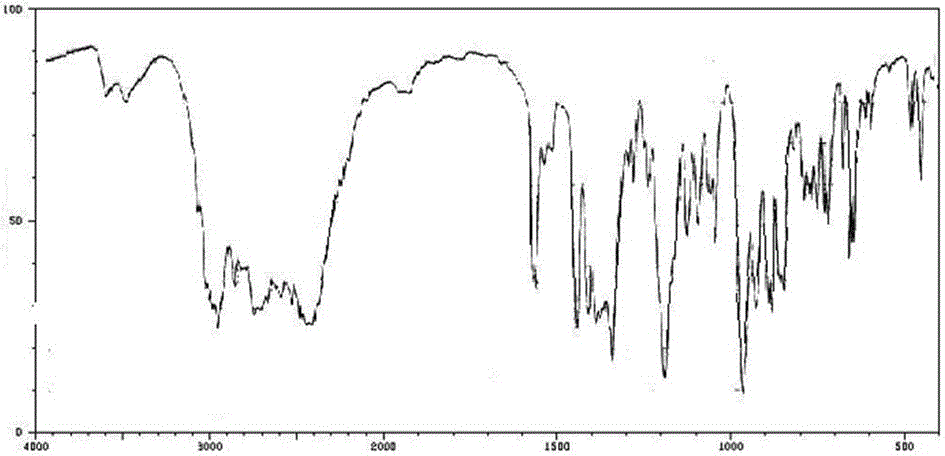
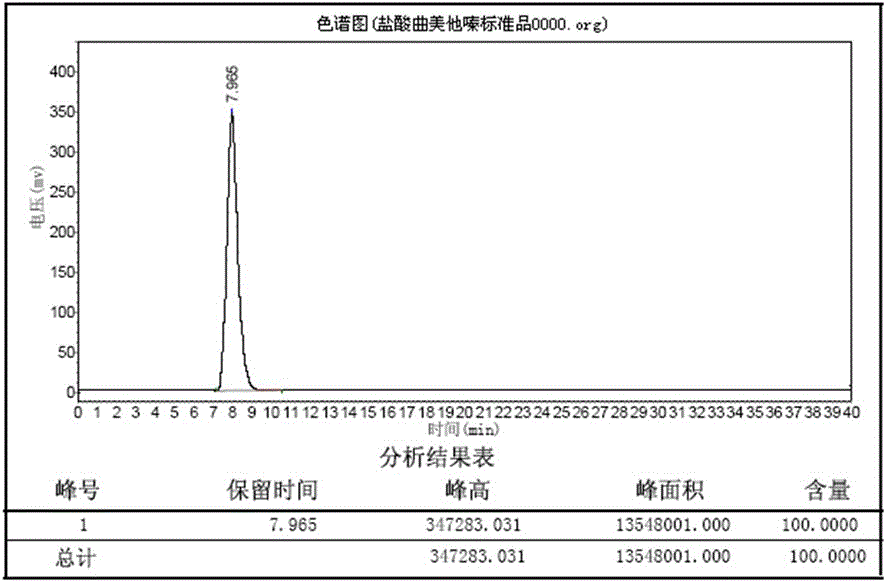
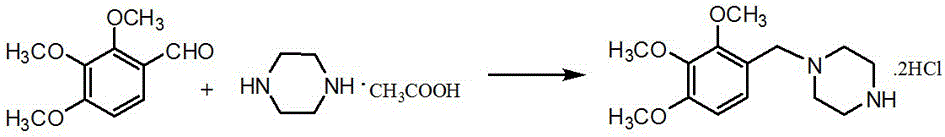Method for preparing trimetazidine dihydrochloride
A technology of trimetazidine hydrochloride and piperazine, which is applied in the field of drug synthesis, can solve the problems of dangerous lithium tetrahydrogen aluminum and 2-piperazinone, which are difficult to obtain and low yield, and achieve simple operation and mild reaction conditions Safe, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Put 300 g of methanol into the hydrogenation kettle, add 52 g (0.6 mol) of piperazine, add 36 g (0.6 mol) of acetic acid under mechanical stirring, heat to 40 ° C, keep for 30 min; cool to 35 ° C, add 2,3 , 130g (0.66mol) of 4-trimethoxybenzaldehyde, 1g (0.0027mol) of hexadecyltrimethylammonium bromide, 2g of nickel catalyst, airtight, nitrogen replacement in the system, keeping the hydrogen pressure at 0.5~0.8MPa, The system temperature was 50-60°C, and the reaction was stirred for 4 hours. Cool, release the pressure, filter to remove the catalyst, add toluene after the filtrate is concentrated, add 50g of 5% sodium hydroxide water, stir for 15min, stand still and separate layers, add 20g of water to the toluene layer to wash once, add 300g of ethanol after concentrating under reduced pressure, and add activated carbon 1 g, stirred for 30 minutes to decolorize, filtered, slowly added hydrochloric acid to the filtrate to adjust the pH to 2, stirred for 30 min...
Embodiment 2
[0042] Put 280g of methanol into the hydrogenation kettle, add 52g (0.6mol) of piperazine, add 36g (0.6mol) of acetic acid under mechanical stirring, heat to 40°C, keep for 30 min; cool to 35°C, add 2,3,4-trimethyl Oxybenzaldehyde 120g (0.61mol), hexadecyltrimethylammonium bromide 0.75g (0.002mol), nickel catalyst 2g, airtight, system nitrogen replacement, keep hydrogen pressure at 0.5~0.8MPa, system temperature at Stir and react for 4 hours at 50~60°C. Cool, release the pressure, filter to remove the catalyst, add toluene after the filtrate is concentrated, add 50g of 5% sodium hydroxide water, stir for 15min, stand still and separate layers, add 20g of water to the toluene layer to wash once, add 300g of ethanol after concentrating under reduced pressure, and add activated carbon 1g, stirred for 30 minutes to decolorize, filtered, slowly added hydrochloric acid to the filtrate to adjust the pH to 2, stirred for 30 minutes, filtered, washed with ethanol, dried to obtain 189.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com