A kind of purification method of imidazoles ionic liquid
A technology of ionic liquid and purification method, which is applied in the field of ionic liquid production, can solve problems such as product yield reduction, achieve the effects of improving product quality, reducing extraction times, and reducing insoluble flocculent impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of purification method of imidazoles ionic liquid, comprises the following steps:
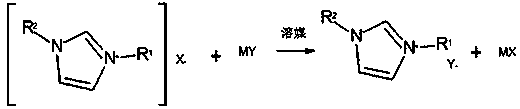
[0037] (1) Under stirring, the crude product [R 1 R 2 IM]X performs anion exchange reaction with the salt MY containing the target anion in aqueous medium to obtain [R 1 R 2 IM] Mixed solution A of Y and MX; R 1 for C 2 ~C 8 One of the alkyl, allyl and ethyl acetate groups; R 2 for C 1 ~C 4 Alkyl; X is halogen or ester; M is H or alkali metal element; Y is BF 4 - 、PF 6 - , SbF 6 - , N(CF 3 SO 2 ) - , N(C 2 f 5 SO 2 ) - , N(FSO 2 ) 2 - or CF 3 SO 3 - ;
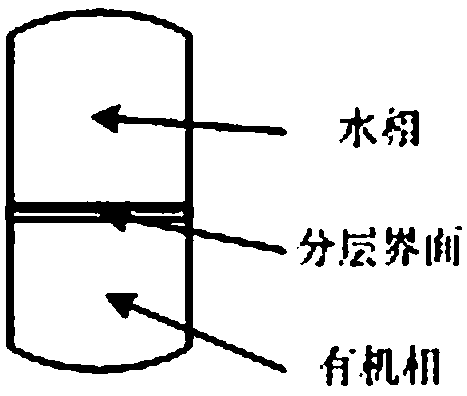
[0038] (2) Add an organic extractant to the mixed solution A in step (1), mix evenly and let it stand, the mixed solution will automatically stratify, containing ionic liquid [R 1 R 2 The organic phase of IM] Y is in the lower floor, and the aqueous phase containing MX is in the upper floor; the organic extractant is at least one of methylene chloride, chloroform, carbon tetrachloride and ether;
[0...
Embodiment 2
[0045] Purification of 1-butyl-3-methylimidazolium tetrafluoroborate
[0046] 1.0 mol of 1-butyl-3-methylimidazolium bromide was dissolved in 500 mL of water, 1.0 mol of ammonium tetrafluoroborate was added with stirring, and the reaction was stirred at room temperature for 24 hours.
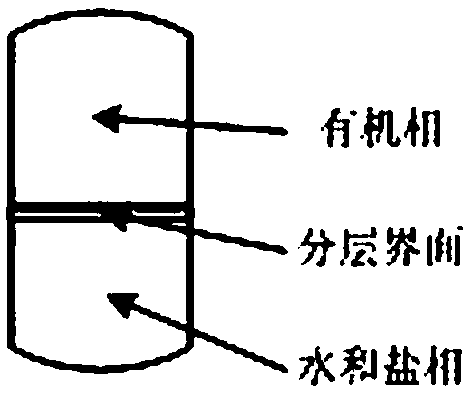
[0047] Add 400mL of dichloromethane to the resulting mixed solution, transfer it to a separatory funnel, add 100g of sodium nitrate, shake it, let it stand, and layer into the organic phase in the upper layer, and the water phase in the lower layer; drain the lower water phase, and add the organic phase again 500mL of water and 150g of sodium nitrate, shake, stand still, separate layers, discharge the lower aqueous phase, and repeat this cleaning operation once again; at this time, it is detected that the organic phase is free of bromide ions and ammonium ions, and a colorless transparent liquid is obtained by vacuum rotary evaporation Product, yield 92.0%.
[0048]
Embodiment 3
[0050] Purification of 1-allyl-3-butylimidazolium hexafluorophosphate
[0051] Dissolve 1.0 mol of 1-allyl-3-butylimidazolium chloride in 500 mL of water, add 1.0 mol of potassium hexafluorophosphate under stirring, and react with stirring at room temperature for 12 hours.
[0052] Add 400mL of dichloromethane to the resulting mixed solution, transfer to a separatory funnel, add 110g of potassium carbonate, oscillate, let stand, and layer into the organic phase in the upper layer, and the water phase in the lower layer; the lower water phase is discharged, and the organic phase is added again. 500mL of water and 180g of potassium carbonate, shake, stand still, separate layers, discharge the lower aqueous phase, and repeat this cleaning operation twice; at this time, the organic phase is detected to be free of bromide ions and ammonium ions, and a light yellow transparent liquid is obtained by vacuum rotary evaporation Product, yield 93.0%.
[0053]
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com