Carbon nitride loaded iron oxide photocatalyst capable of efficiently degrading p-nitrophenol
A technology of p-nitrophenol and photocatalyst, which is applied in the direction of physical/chemical process catalyst, oxidized water/sewage treatment, chemical instruments and methods, etc., to achieve the effect of easy-to-obtain raw materials, excellent photocatalytic performance, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Take 5g of guanidine hydrochloride, transfer it to a crucible, cover and seal it, transfer it to a muffle furnace, raise it from room temperature to 550 °C at a rate of 2.3 °C / min, keep it warm for 4 hours, cool naturally to room temperature, and grind into powder to obtain g-C 3 N 4 nanocatalyst.
Embodiment 2
[0015] Example 2: Weigh 5g of guanidine hydrochloride, transfer it to a crucible, cover and seal it, transfer it to a muffle furnace, raise it from room temperature to 550 °C at a rate of 2.3 °C / min, keep it warm for 4 hours, cool naturally to room temperature, and grind into powder to obtain g-C 3 N 4 nanocatalyst. Weigh 0.097g of Fe(NO 3 ) 3 9H 2 O, dissolved in 50mL isopropanol, stirred for 30min to obtain a uniform solution, 1.3mL of the above solution was added dropwise to 0.5g g-C 3 N 4 , let stand, dry at 60°C, transfer the resulting mixture to a tube furnace, and pass through N 2 (200mL / min), raised from room temperature to 350 °C at a rate of 1 °C / min, kept for 3 hours, and naturally cooled to room temperature to obtain 0.1wt% Fe 2 o 3 / g -C 3 N 4 catalyst.
Embodiment 3-6
[0017] The preparation method is the same as in Example 2, except that Fe 2 o 3 / g -C 3 N 4The loading amount of the catalyst is 0.3wt%, 0.5wt%, 0.7wt%, 0.8wt% in turn.
[0018] The preparation method of the present invention is novel, and raw material is cheap, and preparation process is simple, and yield is higher, and obtained Fe 2 o 3 / g -C 3 N 4 Nanomaterials have good photocatalytic oxidation performance for p-nitrophenol.
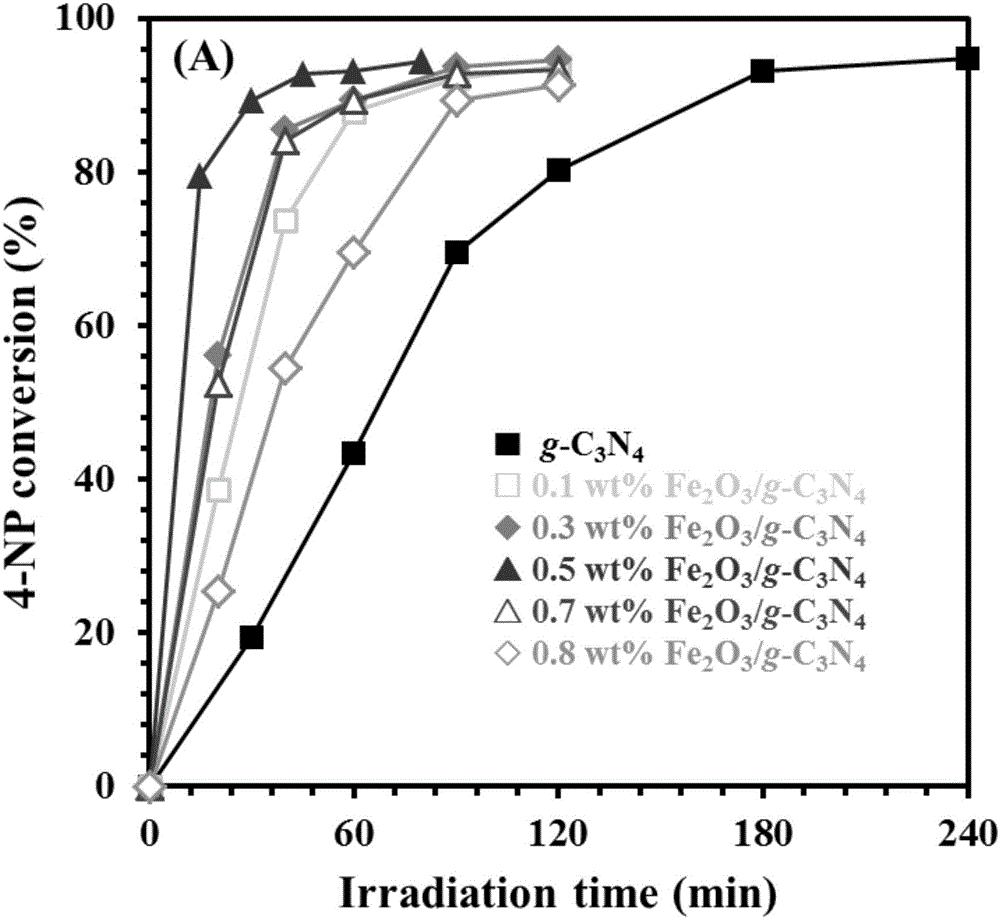
[0019] The obtained Fe of above-mentioned embodiment 2 o 3 / g -C 3 N 4 The XRD spectrum of the sample is shown in figure 1 , where curves (a), (b), (c), (d), (e), (f) are g-C 3 N 4 , 0.1 wt% Fe 2 o 3 / g -C 3 N 4 , 0.3 wt% Fe 2 o 3 / g -C 3 N 4 , 0.5 wt% Fe 2 o 3 / g -C 3 N 4 , 0.7 wt% Fe 2 o 3 / g -C 3 N 4 and 0.8wt% Fe 2 o 3 / g -C 3 N 4 The XRD spectrum; The prepared Fe 2 o 3 / g -C 3 N 4 The TEM pictures of the samples are shown in figure 2 , where graphs (a) and (b) are respectively g-C 3 N 4 The SEM and TEM ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com