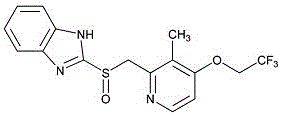
Lansoprazole enteric-coated capsule and preparation method thereof
A technology of lansoprazole enteric and lansoprazole, which is applied in the field of lansoprazole enteric-coated capsule composition and its preparation, can solve problems such as high substance content and poor long-term storage stability, and achieve high storage stability, The effect of low content of related substances and high drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
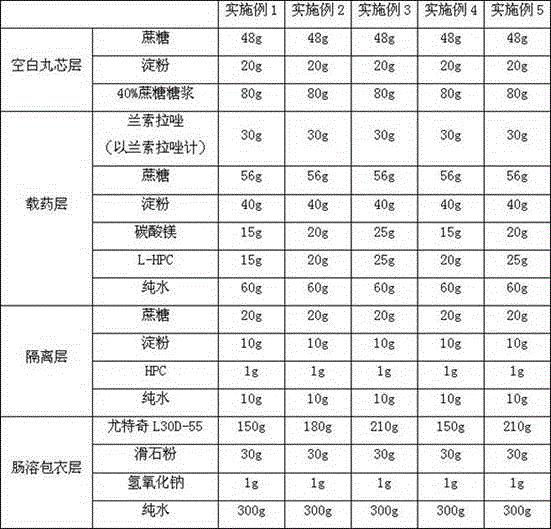
[0107] Embodiment 1-4 Lansoprazole enteric-coated capsule of the present invention
[0108] prescription:
[0109]
[0110] Note: In Examples 1-3, the weight ratio of magnesium carbonate and L-HPC in the drug-loaded layer is 1:1.
[0111] Preparation:
[0112] (1) Preparation of blank pellet cores
[0113] Using coating granulator and atomizing spray technology, sucrose, starch and 40% sucrose syrup (adhesive) are used to make 24-30 mesh blank pellet cores;
[0114] (2) Preparation of drug-loaded pellets
[0115] Dissolve the lansoprazole, sucrose, starch, magnesium carbonate, and L-HPC of the drug-loaded layer in pure water to obtain a drug-containing solution; use a multifunctional granulation bed coater to spray the resulting drug-containing solution on the aforementioned blank The surface of the pellet core is dried and cooled to obtain 14-25 mesh drug-loaded pellets;
[0116] (3) Preparation of isolation pellets
[0117] The sucrose, starch, and HPC of the isolat...
experiment example
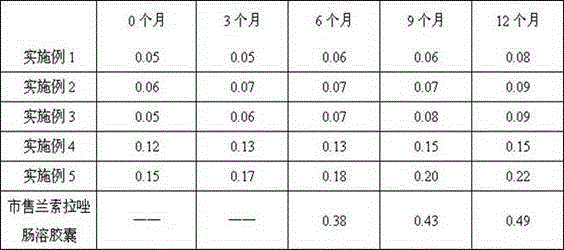
[0122] Experimental example Lansoprazole enteric-coated capsules quality and storage stability research
[0123] Get the lansoprazole enteric-coated capsules that embodiment 1-4 makes, commercially available lansoprazole enteric-coated capsules, and the lansoprazole enteric-coated pellet capsules prepared by CN104523648A embodiment 1, at 40 ℃ ± 2 ℃, relative humidity of 75% ± 5% stored for 12 months, the test results of the percentage of related substances (%) are as follows:
[0124]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com