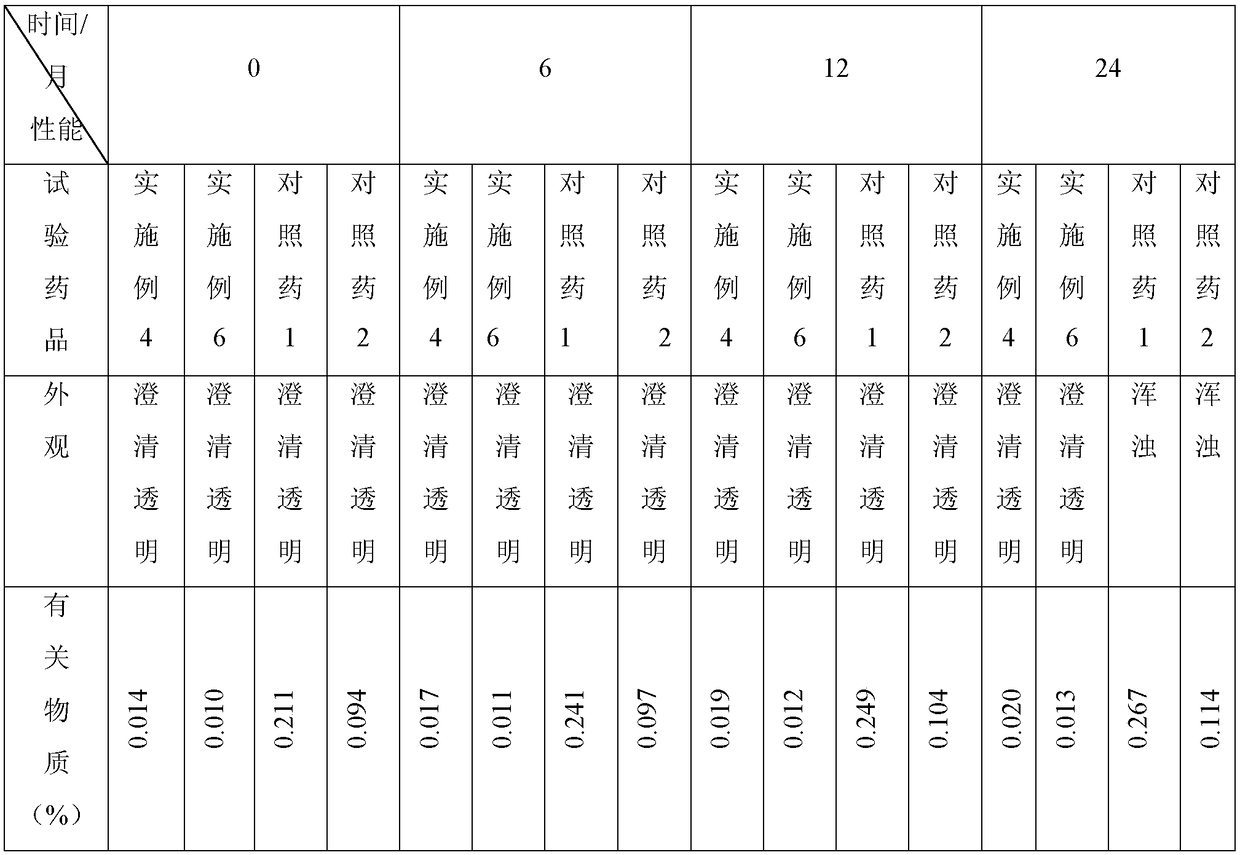
A kind of milrinone lactate composition
A technology of lactic acid milrinone and a composition, applied in the field of medicine, can solve the problems of complex preparation method of lactic acid milrinone freeze-dried powder injection, unsuitable for large-scale industrial production, long production cycle, etc., and is suitable for large-scale production. , The effect of low production cost and low content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 milrinone crystal
[0034] (1) Mix and dissolve the crude milrinone, ethanol, acetone and water at a mass volume ratio of 7g: 83ml: 57ml: 105ml, slowly heat to 94°C, fully stir and dissolve evenly;
[0035] (2) add active carbon decolouring 4.5 hours then, the quality of active carbon is 8% of milrinone crude product, suction filtration while hot, remove active carbon;
[0036] (3) The solution obtained by suction filtration was stirred at a stirring rate of 6 rpm, cooled to 34° C., and the cooling rate was 2° C. / min. When the filtrate was lowered to room temperature, deionized water was added dropwise to it under an ultrasonic field, until the crystals are precipitated;
[0037] (4) Turn off the ultrasonic field, let stand for 27 hours, filter, wash with ethanol, and dry to obtain the milrinone crystals.
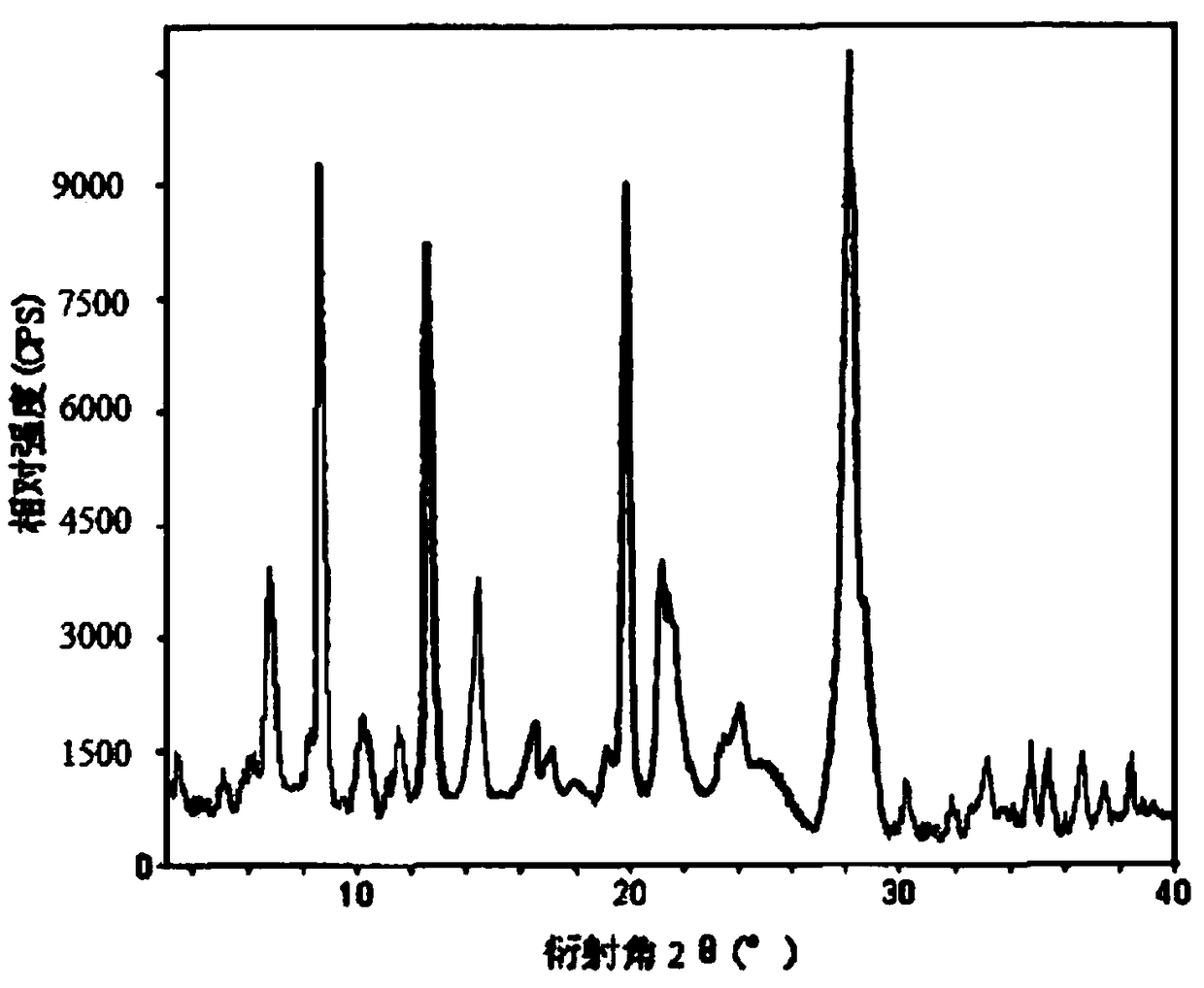
[0038] The obtained milrinone crystal uses Cu-Kα ray to measure the X-ray powder diffraction spectrum that obtains as figure 1 sho...
Embodiment 2
[0039] The preparation of embodiment 2 milrinone crystals
[0040] (1) Mix and dissolve the crude milrinone, ethanol, acetone and water at a mass volume ratio of 6g: 80ml: 55ml: 100ml, slowly heat to 90°C, stir well and dissolve evenly;
[0041] (2) Then add activated carbon for decolorization for 4 hours, the quality of activated carbon is 7% of the crude product of milrinone, suction filtration while hot, removes activated carbon;
[0042](3) The solution obtained by suction filtration was stirred at a stirring rate of 5 rpm, cooled to 32° C., and the cooling rate was 2° C. / min. When the filtrate was lowered to room temperature, deionized water was added dropwise thereto under an ultrasonic field, until the crystals are precipitated;
[0043] (4) Turn off the ultrasonic field, let stand for 26 hours, filter, wash with ethanol, and dry to obtain the milrinone crystals.
[0044] The X-ray powder diffraction spectrum obtained by measuring the obtained milrinone crystals using...
Embodiment 3
[0046] (1) Mix and dissolve the crude milrinone, ethanol, acetone and water at a mass volume ratio of 8g: 85ml: 60ml: 110ml, slowly heat to 98°C, fully stir and dissolve evenly;
[0047] (2) add activated carbon decolouring 5 hours then, the quality of activated carbon is 9% of milrinone crude product, suction filtration while hot, removes activated carbon;
[0048] (3) The solution obtained by suction filtration was stirred at a stirring rate of 9 rpm, cooled to 35° C., and the cooling rate was 3° C. / min. When the filtrate was lowered to room temperature, deionized water was added dropwise thereto under an ultrasonic field, until the crystals are precipitated;
[0049] (4) Turn off the ultrasonic field, let stand for 28 hours, filter, wash with ethanol, and dry to obtain the milrinone crystals.
[0050] The X-ray powder diffraction spectrum obtained by measuring the obtained milrinone crystals using Cu-Kα rays is basically consistent with that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com