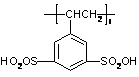
A kind of method for preparing anhydrous peroxy organic carboxylic acid solution
A technology of anhydrous peroxy organic carboxylic acid and organic carboxylic acid, applied in the direction of organic chemistry, peroxygen compound preparation, chemical instruments and methods, etc., can solve the problem of long rectification time, reduction of device production capacity, and consumption of large circulating cooling water and other problems, to achieve high reactivity and thermal stability, reduce by-product production, and save steam consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of peracetic acid solution
[0050] First, 100 g of bissulfonic acid-based polystyrene resin was charged into a reactor with a volume of 2 L, and then 600 g of acetic acid and 440 g of ethyl acetate were added into the reactor.
[0051] The reactor has a packed distillation column and a reflux condenser with a settling tank. Stir this solution at about 20kPa (absolute pressure), heat it to about 40°C with steam, and add a total amount of 220g of 50% (by weight) hydrogen peroxide solution into the reactor. Control the temperature of the reactor at about 40-45°C, introduce the organic phase in the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser into the adsorption tower, which is equipped with molecular sieves, and the organic phase passes through the molecular sieves Adsorption dehydration, the water content is reduced to below 1000ppm (mass), and returned to the rectification tower; the water phase is continuously dra...
Embodiment 2
[0060] Preparation of peroxypropionic acid solution
[0061] 100g bissulfonic acid polystyrene resin is charged in advance in the reactor that volume is 2L, then 600g propionic acid, 400g ethyl propionate and 1kg tributyl phosphate are charged in the reactor. Stir this solution at about 10kPa (absolute pressure), heat it to about 50°C with steam, and add a total of 220g of 50% (weight) hydrogen peroxide aqueous solution into the reactor. Control the reaction temperature at about 50-55°C, introduce the organic phase in the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser into the adsorption tower, the adsorption tower is equipped with molecular sieves, and the organic phase is dehydrated by molecular sieve adsorption , the water content drops below 1000ppm (mass), and returns to the rectifying tower; the water phase is continuously drained and the organic solvent is recovered through the organic solvent recovery device and returned to the ...
Embodiment 3
[0071] Preparation of long-term peroxypropionic acid solution
[0072] In advance, 100 g of bissulfonic acid-based polystyrene resin was loaded into a reactor with a volume of 2 L, and then 600 g of propionic acid, 400 g of ethyl propionate and 1 kg of tributyl phosphate were loaded into the reactor. Stir this solution at about 10kPa (absolute pressure), heat it to about 50°C with steam, and add a total of 220g of 50% (weight) hydrogen peroxide aqueous solution into the reactor. Control the reaction temperature at about 50-55°C, introduce the organic phase in the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser into the adsorption tower, the adsorption tower is equipped with molecular sieves, and the organic phase is dehydrated by molecular sieve adsorption , the water content drops below 1000ppm (mass), and returns to the rectifying tower; the water phase is continuously drained and the organic solvent is recovered by the organic solvent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com