A method for detecting enantiomers in vildagliptin intermediates
A technology of enantiomers and intermediates, applied in the field of high-efficiency liquid phase detection of enantiomers in -1--2-pyrrolidinecarbonitrile, can solve the problem of no detection method and peak shape symmetry Not good, can not be improved and other problems, to achieve good effect, strong practicability, easy to adjust the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] S3, the preparation of mixed solution:
[0045] Weigh (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile into a volumetric flask, and accurately measure (2R)-1-(2-chloroacetyl)-2- Add the pyrrolidinecarbonitrile reference substance solution into a volumetric flask and dilute to the mark with mobile phase, mix and shake well, and use it as a mixed solution for later use. According to the mass volume ratio (g / L), the ratio of (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile:mobile phase is 0.1~5:1, and the optimal ratio is 1:1; According to the mass volume ratio (g / L), the ratio of (2R)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile:mobile phase is 0.001~0.1:1, and the optimal ratio is 0.01:1;
[0046] S4, draw equal amount of reference substance solution, need testing solution and mixed solution respectively, inject in the high-efficiency chromatograph and measure, and the determination condition of described high-efficiency liquid chromatography comprises:
[0047] Insp...
Embodiment 1
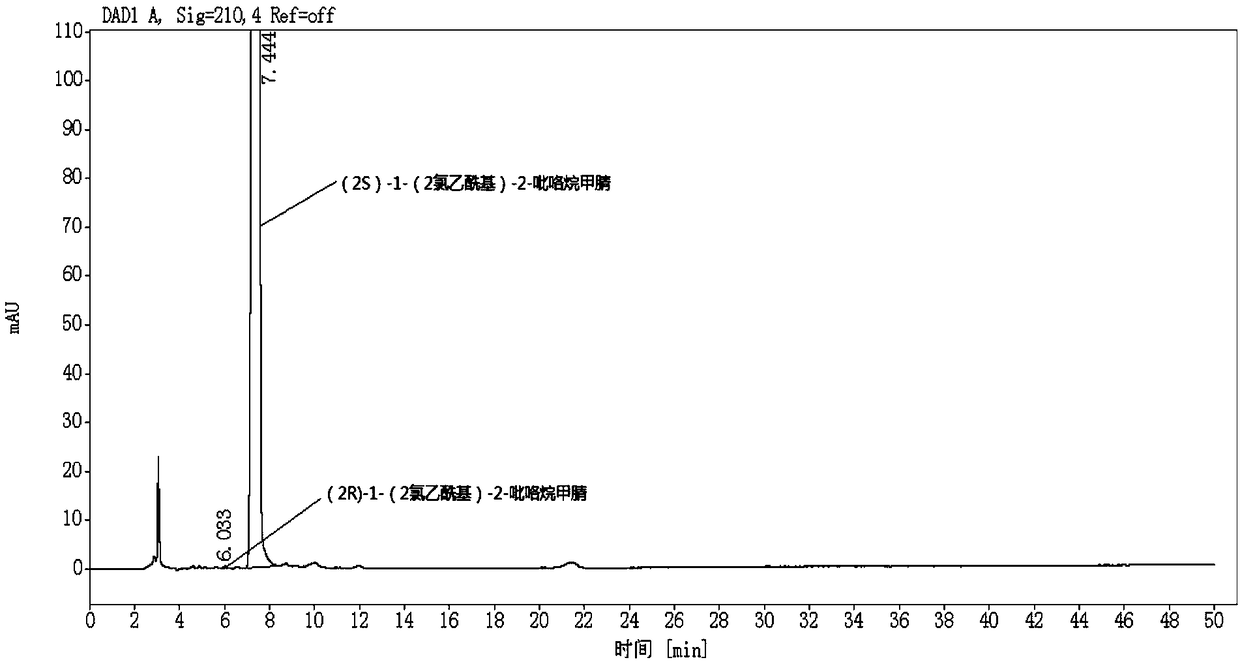
[0057] Choose the (2S)-1-(2-chloroacetyl group)-2-pyrrolidine carbonitrile sample that is 160104 batch number, its (2R)-1-(2-chloroacetyl group)-2-pyrrolidine carbonitrile content is detected, including the following steps:
[0058] S1. Preparation of (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile test solution:
[0059] Weigh 25mg of (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile sample and add 25mL of mobile phase, after mixing evenly, it is formulated into a solution containing 1.0mg of vildagliptin per 1mL as the test solution Product solution, ready to use.
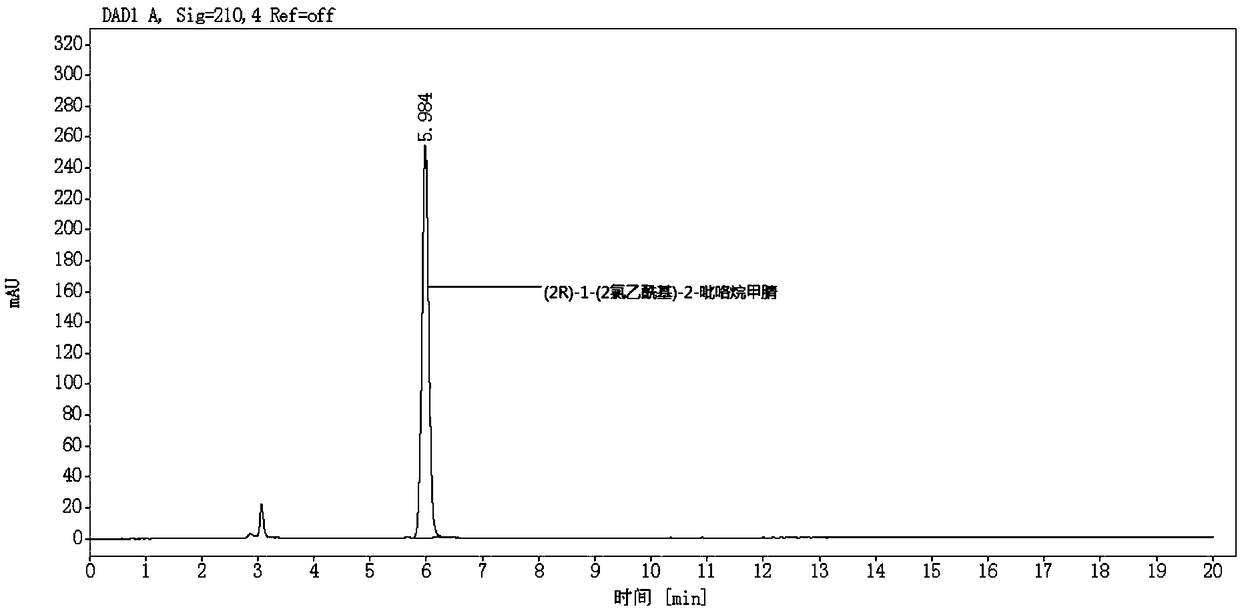
[0060] S2. Preparation of (2R)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile reference substance solution:
[0061] Weigh an appropriate amount of (2R)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile reference substance, weigh it accurately, dissolve the mobile phase and quantitatively dilute it to make a solution containing about 100 μg per 1 mL, as (2R )-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile reference s...
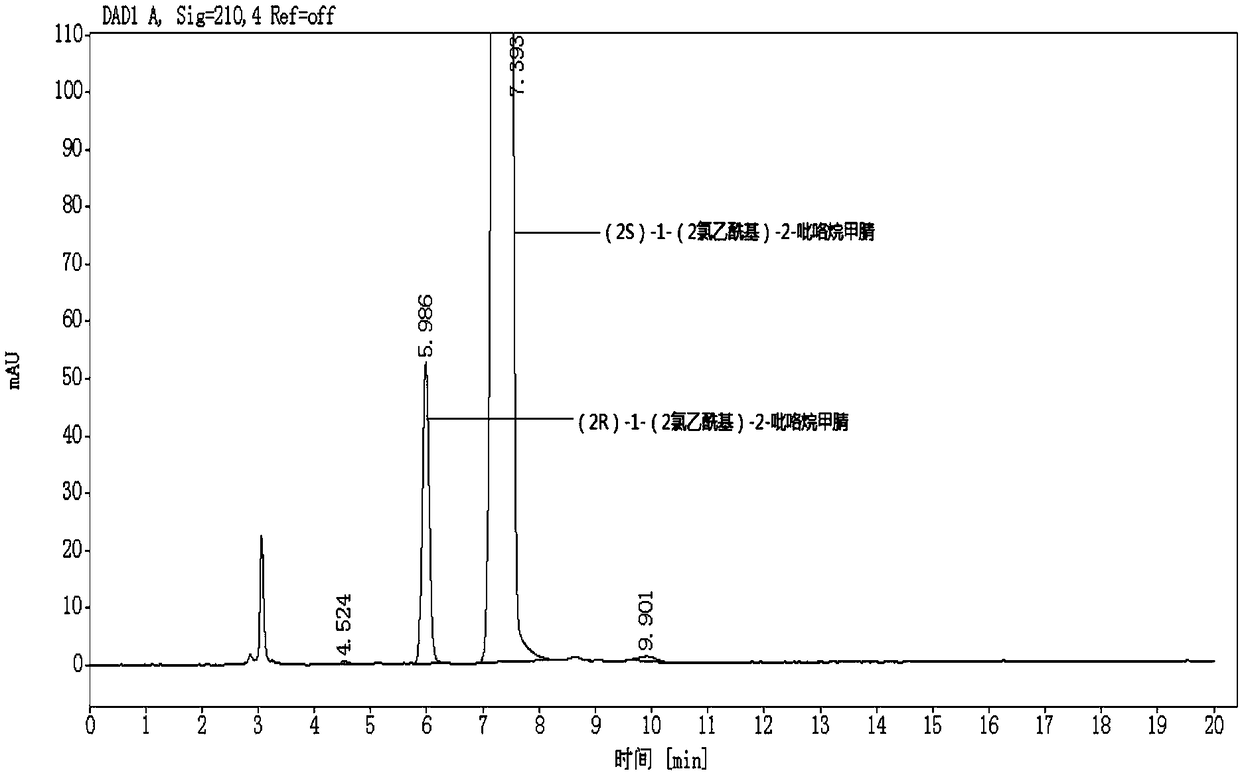
Embodiment 2
[0073] Choose the (2S)-1-(2-chloroacetyl)-2-pyrrolidine carbonitrile sample of the same batch as in Example 1, to (2R)-1-(2-chloroacetyl)-2-pyrrole The content of alkanecarbonitrile is detected, the difference between this embodiment and embodiment 1 is only: the parameter of flow velocity in the chromatographic condition is different, and other detection conditions are consistent with embodiment 1. In this embodiment, the assay conditions of high performance liquid chromatography include:
[0074] Chromatographic column: CHIRALPAK IC (model: length 250mm, inner diameter 4.6mm, cellulose surface covalently bonded silica filler, filler particle size 5μm);
[0075] Detector: UV detector;
[0076] Detection wavelength: 210nm;
[0077] Column temperature: 30°C;
[0078] Flow rate: 0.8mL / min;
[0079] Mobile phase: Calculated by volume ratio, n-butanol:ethanol:diethylamine=60:40:0.05.
[0080] It can be seen through detection that the content of (2R)-1-(2-chloroacetyl)-2-pyrro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com