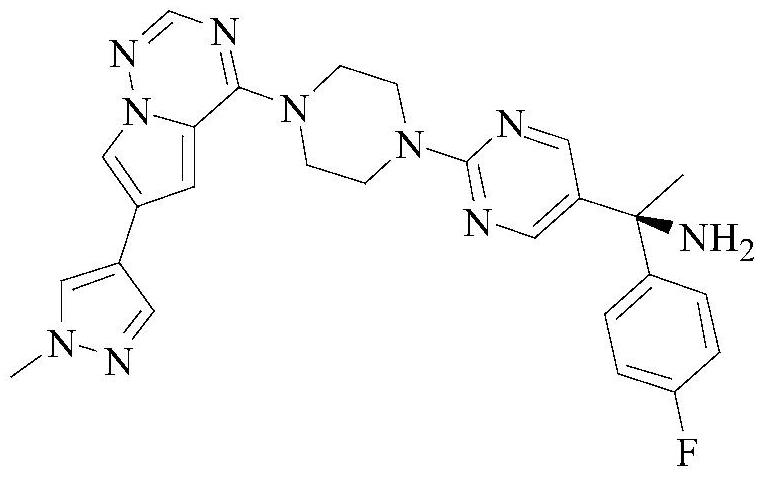
A method for detecting enantiomers in avapritinib intermediates
A technology for enantiomers and intermediates, applied in the field of detection of enantiomers in Avapritinib intermediates, to achieve the effect of strong practicability, good detection effect, and simple detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
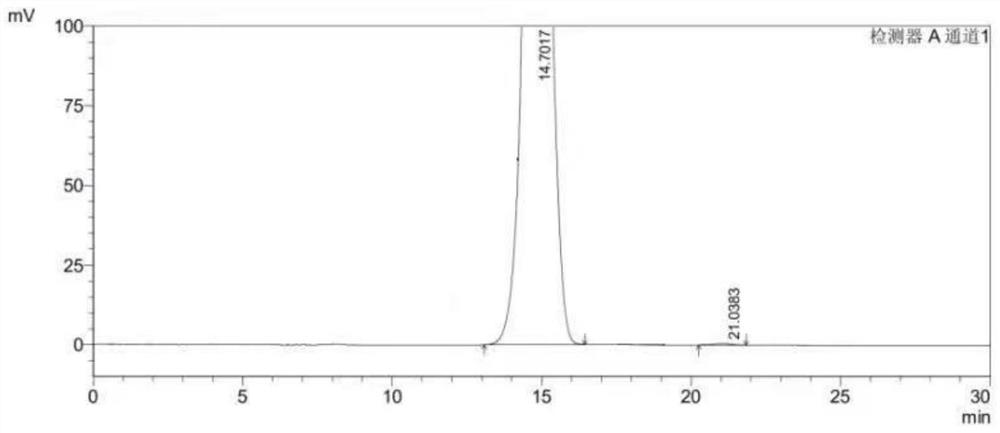
[0048] Select the content of (S)-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl)pyrimidin-5-yl]ethylamine whose batch number is 20210421 for detection, including the following steps:
[0049] S1. Preparation of (S)-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl)pyrimidin-5-yl]ethylamine test solution:
[0050] Weigh 10mg of (S)-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl)pyrimidin-5-yl]ethanamine sample and add 10mL n-hexane-absolute ethanol solution, by It is prepared by mixing n-hexane and absolute ethanol with a volume ratio of 1:1. After mixing evenly, it is prepared to contain (S)-1-(4-fluorophenyl)-1-[2-(piperazine-1 -yl) pyrimidin-5-yl] ethylamine 1.0mg solution, as need testing solution, stand-by.
[0051] S2. Preparation of (R)-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl)pyrimidin-5-yl]ethylamine reference substance stock solution:
[0052] Weigh (R)-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl)pyrimidin-5-yl]ethylamine, accurately weigh 100mg, n-hexane-absolute ethanol solution Dissolve and...
Embodiment 2
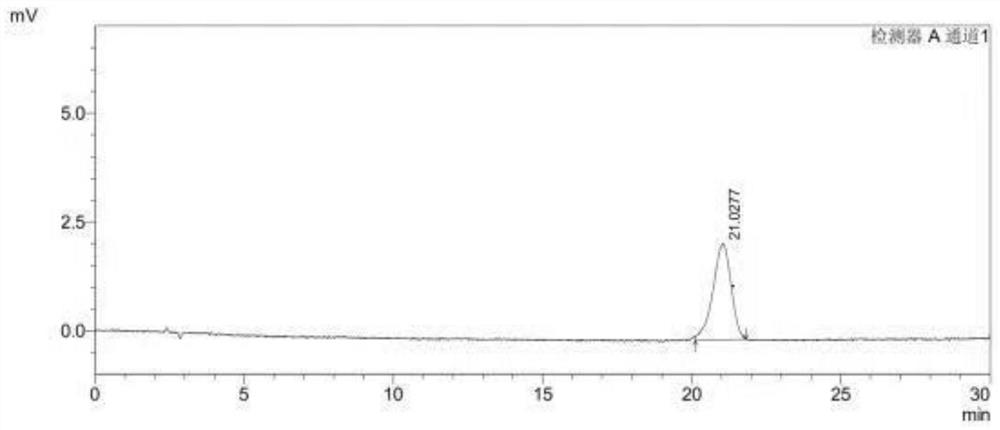
[0065] Choose the (S)-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl) pyrimidin-5-yl] ethylamine sample of the same batch as Example 1, to (R )-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl)pyrimidin-5-yl]ethanamine peak purity is detected, the difference between the present embodiment and embodiment 1 is only : different detectors are used in the chromatographic conditions, and other detection conditions are consistent with embodiment 1. In this embodiment, the assay conditions of high performance liquid chromatography include:
[0066] Chromatographic column: CHIRALCEL*OD-H (model: length 250mm, inner diameter 4.6mm, cellulose surface covalently bonded silica gel filler, filler particle size 5μm);
[0067] Detector: DAD detector;
[0068] Detection wavelength: 220nm;
[0069] Column temperature: 30°C;
[0070] Flow rate: 1.0mL / min;
[0071] Mobile phase: Calculated by volume ratio, n-hexane:ethanol:isopropanol=99:0.5:0.5. It can be seen from the test that the peak purity of (S)-1-(...
Embodiment 3
[0073] Select (R)-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl)pyrimidin-5-yl]ethanamine reference substance solution for detection limit and quantification limit detection, signal-to-noise ratio 10:1 is the quantification limit, and the signal-to-noise ratio is 3:1 is the detection limit. The detection conditions of this embodiment are consistent with those of Embodiment 1. In this embodiment, the assay conditions of high performance liquid chromatography include:
[0074] Chromatographic column: CHIRALCEL*OD-H (model: length 250mm, inner diameter 4.6mm, cellulose surface covalently bonded silica gel filler, filler particle size 5μm);
[0075] Detector: UV detector;
[0076] Detection wavelength: 220nm;
[0077] Column temperature: 30°C;
[0078] Flow rate: 1.0mL / min;
[0079] Mobile phase: Calculated by volume ratio, n-hexane:isopropanol:triethylamine=80:20:0.05.
[0080] After testing, it can be seen that the detection limit of (R)-1-(4-fluorophenyl)-1-[2-(piperazin-1-yl)py...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com