Star-shaped hydroxyl polyester adopting polyhydric alcohol as nucleus and preparation method and application of star-shaped hydroxyl polyester
A technology of hydroxyl polyester and polyol, applied in polyurea/polyurethane coatings, coatings, etc., can solve the needs of shortening the activation period of two-component polyurethane coatings, environmental and human health hazards, and the difficulty of applying star-shaped hydroxyl polyesters to environmental protection And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
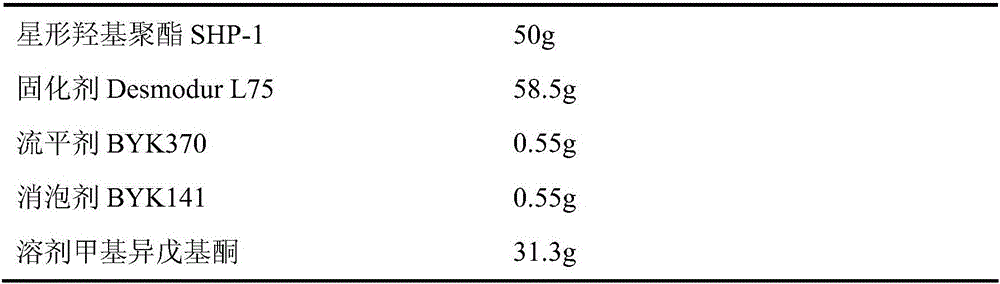
Examples
Embodiment 1
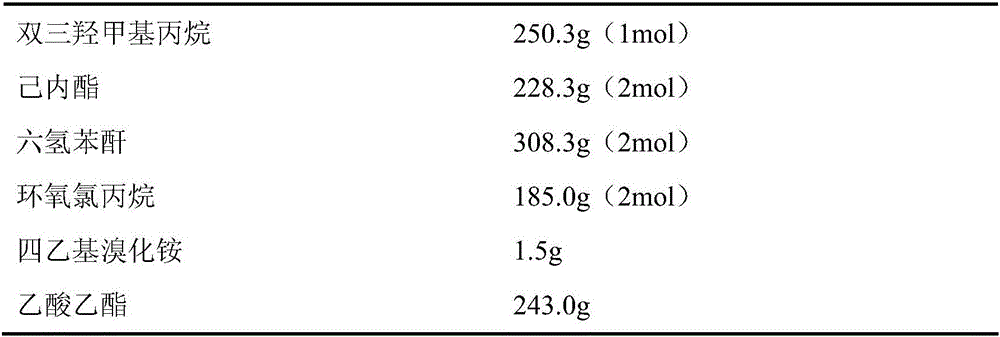
[0039] 1. Raw material composition
[0040] See Table 1 for the raw materials and proportions used in the preparation of the star-shaped hydroxyl polyester SHP-1.
[0041] Table 1
[0042]
[0043] 2. Preparation
[0044] The preparation of star-shaped hydroxyl polyester SHP-1 specifically comprises the steps:
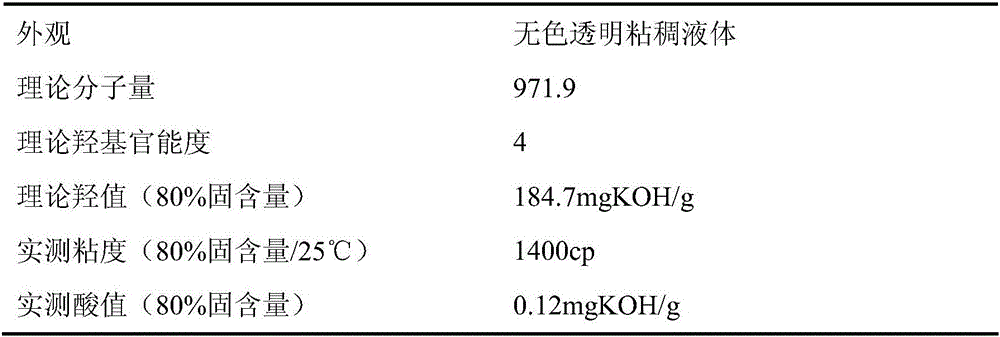
[0045] (1) Add 250.3g of ditrimethylolpropane, 228.3g of caprolactone, and 308.3g of hexahydrophthalic anhydride to a four-necked flask equipped with a mechanical stirrer, a thermometer, a spherical condenser, and a nitrogen port. After reacting for 7 hours under the protection of nitrogen, the infrared characteristic peak of caprolactone in the infrared spectrum detection reaction system disappeared, and the acid value of the detection reaction system dropped to 50% (mass percentage) of its initial value, and the matrix star polyester was obtained by cooling.
[0046] (2) Add 185.0g epichlorohydrin and 1.5g tetraethylammonium bromide to the matrix star polyester...
Embodiment 2
[0063] 1. Raw material composition
[0064] See Table 5 for the raw materials and proportions used in the preparation of the star-shaped hydroxyl polyester SHP-2.
[0065] table 5
[0066]
[0067]
[0068] 2. Preparation
[0069] The preparation of star-shaped hydroxyl polyester SHP-2 specifically comprises the following steps:
[0070] (1) In a four-necked flask equipped with a mechanical stirrer, a thermometer, a spherical condenser, and a nitrogen port, add 250.3g of ditrimethylolpropane, 228.3g of caprolactone, and 296.2g of phthalic anhydride at 110 ℃, reaction under nitrogen protection for 8h, the infrared characteristic peak of caprolactone in the infrared spectrum detection reaction system disappeared, the acid value of the detection reaction system dropped to 50% (mass percentage) of its initial value, and the matrix star polyester was obtained by cooling.
[0071] (2) Add 436g of benzyl glycidyl ether and 2.5g of tetraethylammonium bromide to the matrix star ...
Embodiment 3
[0085] 1. Raw material composition
[0086] See Table 9 for the raw materials and proportions used in the preparation of the star-shaped hydroxyl polyester SHP-3.
[0087] Table 9
[0088]
[0089] 2. Preparation
[0090] The preparation of star-shaped hydroxyl polyester SHP-3 specifically comprises the steps:
[0091] (1) Add 134.2g trimethylolpropane, 114.1g caprolactone, and 308.3g hexahydrophthalic anhydride to a four-necked flask equipped with a mechanical stirrer, a thermometer, a spherical condenser, and a nitrogen port. After reacting for 8 hours under protection, the infrared characteristic peak of caprolactone in the reaction system detected by infrared spectroscopy disappeared, and the acid value of the detected reaction system dropped to 50% (mass percentage) of its initial value, and the matrix star polyester was obtained by cooling down.
[0092] (2) Add 185.0g epichlorohydrin and 1.5g tetrabutylammonium chloride to the matrix star polyester prepared in ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com