Preparation method of right-handed lansoprazole crystal form
A dexlansoprazole crystal and a technology for dexlansoprazole are applied in the field of preparation of dexlansoprazole crystal forms, and can solve the problem that the consumption of the solvent is too large, the solvent recovery is difficult, and n-heptane The problem of large dosage, etc., can achieve the effect of reducing solvent consumption, high production efficiency and low environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) 10g lansoprazole hydrate (1.5 crystalline waters) after the purification is dissolved in 50ml ethyl acetate, adds saturated sodium bicarbonate solution, stirs, separates phases, and the aqueous phase is extracted once with ethyl acetate again, Combine the organic phases, add anhydrous sodium sulfate and activated carbon to the organic phase, stir, filter, and wash with ethyl acetate.
[0030] (2) Combine the filtrate and washing liquid, and slowly add 100ml of isopropyl ether under stirring at room temperature, then add seed crystal A, and stir.
[0031] (3) When the solution is cooled to 0-10°C, solids will precipitate out soon. Stir at this temperature for 0.5-1 h.
[0032] (4) Suction filtration, the filter cake is washed with an appropriate amount of isopropyl ether.
[0033] (5) The product was vacuum-dried at 40° C. for 24 h.
[0034] Obtained 8.2 g of dense white crystals with a yield of 81%. Melting point: 142-143°C.
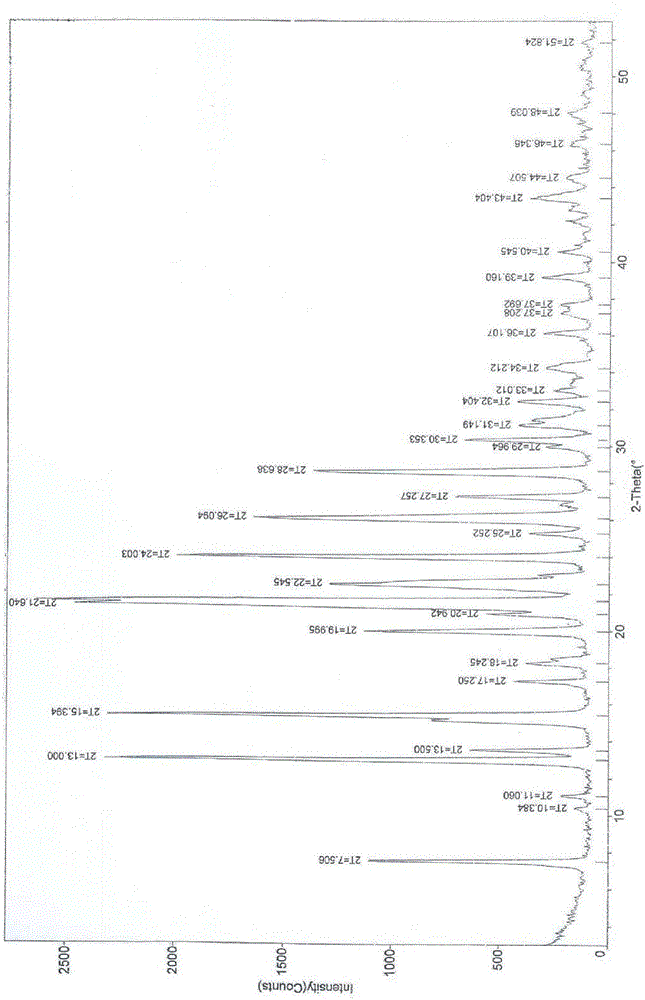
[0035] figure 1 Be the XRD figure...
Embodiment 2
[0040] (1) 20g lansoprazole hydrate (1.5 crystalline waters) after the purification is dissolved in 100ml ethyl acetate, adds saturated sodium bicarbonate solution, stirs, separates phases, and the aqueous phase is extracted once with ethyl acetate again, Combine the organic phases, add anhydrous sodium sulfate and activated carbon to the organic phase, stir, filter, and wash with ethyl acetate.
[0041] (2) Combine the filtrate and washing liquid, and slowly add 100ml of isopropyl ether under stirring at room temperature, then add seed crystal A, and stir.
[0042] (3) When the solution is cooled to 5°C, a solid precipitates out soon. Stir at this temperature for 0.8h.
[0043] (4) Suction filtration, the filter cake is washed with an appropriate amount of isopropyl ether.
[0044] (5) The product was vacuum-dried at 40° C. for 24 hours to obtain 16.5 g of dense white crystals with a yield of 82.5%.
Embodiment 3
[0046] (1) 30g lansoprazole hydrate (1.5 crystal waters) after the purification is dissolved in 150ml ethyl acetate, adds saturated sodium bicarbonate solution, stirs, separates phases, and the aqueous phase is extracted once with ethyl acetate again, Combine the organic phases, add anhydrous sodium sulfate and activated carbon to the organic phase, stir, filter, and wash with ethyl acetate.
[0047] (2) Combine the filtrate and washing liquid, and slowly add 100ml of isopropyl ether under stirring at room temperature, then add seed crystal A, and stir.
[0048] (3) When the solution is cooled to 3°C, a solid precipitates out soon. Stir at this temperature for 1 h.
[0049] (4) Suction filtration, the filter cake is washed with an appropriate amount of isopropyl ether.
[0050] (5) The product was vacuum-dried at 40° C. for 24 h. 23.8 g of dense white crystals were obtained, with a yield of 79.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com