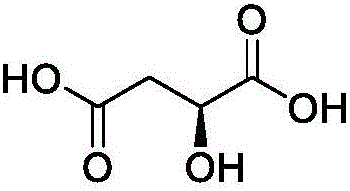
Refining method for L-malic acid
A refining method, malic acid technology, applied in the field of medicine and chemical industry, to achieve the effect of mild conditions, good reproducibility and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
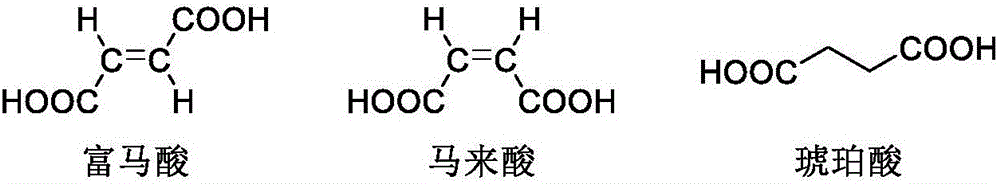
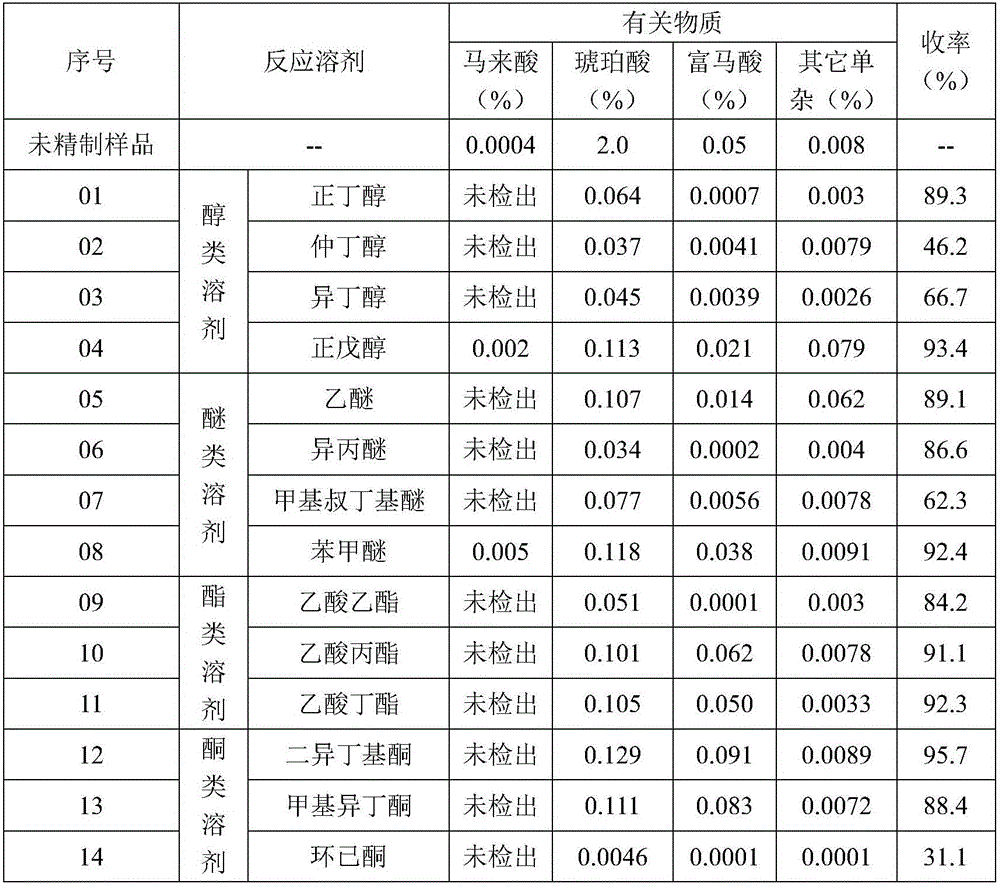
[0059] Add 2.5kg L-malic acid (pharmaceutical excipient grade) and 12.5kg purified water into a clean and dry 50L glass reactor, stir at 28°C and 125r / min to dissolve. Add 20.1kg of isopropyl ether, stir and extract at 165r / min for 0.5h, then let stand for 0.5h to separate the liquid, and collect the lower aqueous phase. Add the water phase to a 50L glass reactor, add 20.1kg of isopropyl ether, stir and extract at 165r / min for 0.5h, then let it stand for 0.5h to separate the liquid, collect the lower layer of water phase, take about 5ml of the sample and concentrate it to dryness under reduced pressure before sending it to HPLC for inspection , Maleic acid, 0.035% succinic acid, and 0.0018% fumaric acid were not detected in the monitoring water phase. Suction filtration with a 0.45 μm filter membrane, and the filtrate was rotary evaporated at 60°C to 65°C until no liquid dripped out of the condenser, and continued rotary evaporation for 0.5h. Add 10.05 kg of isopropyl ether i...
Embodiment 2
[0061] Add 5kg of L-malic acid (pharmaceutical excipient grade) and 25kg of purified water into a clean and dry 100L glass reactor, stir at 20°C to 40°C and 130r / min to dissolve. Add 50kg of ethyl acetate, stir and extract at 130r / min for 0.5h, then let stand for 0.5h to separate the liquid, and collect the lower aqueous phase. Add the water phase to a 100L glass reactor, add 50kg of ethyl acetate, stir and extract at 130r / min for 0.5h, then let stand for 0.5h to separate the liquid, collect the lower layer of water phase, take about 5ml of the sample and concentrate it to dryness under reduced pressure, then send it to HPLC for inspection. No maleic acid, 0.051% succinic acid, and 0.0009% fumaric acid were detected in the monitoring water phase. Suction filtration with a 0.45 μm filter membrane, and the filtrate was rotary evaporated at 70°C to 75°C until no liquid dripped out of the condenser, and continued rotary evaporation for 0.5h. Add 25 kg of ethyl acetate into the ro...
Embodiment 3
[0063] Add 2kg of L-malic acid (pharmaceutical excipient grade) and 10kg of purified water into a clean and dry 50L glass reactor, stir at 26°C and 142r / min to dissolve. Add 17.96kg of n-butanol, stir and extract at 142r / min for 0.5h, then let stand for 0.5h to separate the liquid, and collect the lower aqueous phase. Then add the water phase into a 50L glass reactor, add 17.96kg of n-butanol, stir and extract at 142r / min for 0.5h, then let it stand for 0.5h to separate the liquid, collect the lower water phase, take about 5ml of the sample and concentrate it to dryness under reduced pressure, then send it to HPLC for inspection , Maleic acid, 0.064% succinic acid, and 0.0005% fumaric acid were not detected in the monitoring water phase. Suction filtration with a 0.45 μm filter membrane, and the filtrate was rotary evaporated at 70°C to 75°C until no liquid dripped out of the condenser, and continued rotary evaporation for 0.5h. Add 8.98 kg of n-butanol into the rotary evapor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com