Thermo-sensitive coding microsphere carrier for drug controlled release and preparation method of microsphere carrier
A technology of drug controlled release and temperature sensitivity, which is applied in the fields of pharmaceutical formulation, drug delivery, liquid delivery, etc. It can solve the problems of uneven particle size and pore size of microspheres, reduce biological safety, and difficulty in residue determination, etc. The effect of drug rate, low cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of NIPAM / acrylamide drug controlled release microsphere carrier:
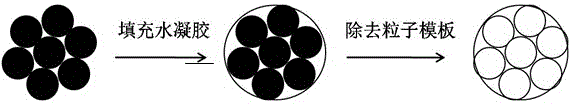
[0035] 1. Preparation of ordered colloidal crystal microspheres: monodisperse SiO 2 Add deionized water to the nanoparticles to adjust the concentration to 20%-30% (w / v); use a glass microfluidic device to shear the colloidal solution into monodisperse droplets in the mobile phase, and place the droplet template in an oven for 80 °C to dry and solidify, remove surface or internal impurities, and place in a muffle furnace for calcination at 800 °C for 3 hours.
[0036]. NIPAM / acrylamide hydrogel filling: firstly, the prepared SiO 2 The colloidal crystal microspheres were subjected to hydrophilic treatment, soaked in a mixture of 70% concentrated sulfuric acid and 30% hydrogen peroxide for 12 hours to make the surface of the microspheres hydroxylated. After repeated cleaning with ultrapure water and drying with nitrogen, the colloidal crystal microspheres were immediately soaked i...
Embodiment 2
[0038] Example 2 Preparation of NIPAM / PEGDA drug controlled release microsphere carrier:
[0039] 1. Preparation of ordered colloidal crystal microspheres Preparation of ordered colloidal crystal microspheres: Add monodisperse PS nanoparticles to deionized water to adjust the concentration to 20%-30% (w / v); The solution was sheared into monodisperse droplets in the mobile phase, and the droplet template was dried and solidified in an oven at 80°C. After removing surface or internal impurities, it was calcined in a muffle furnace at 800°C for 3h.
[0040] . NIPAM / PEGDA hydrogel filling: firstly, the prepared PS colloidal crystal microspheres were hydrophilically treated, soaked in a mixture of 70% concentrated sulfuric acid and 30% hydrogen peroxide for 12 hours to hydroxylate the surface of the microspheres. After repeatedly washing with ultrapure water and blowing dry with nitrogen, the colloidal crystal microspheres can be soaked in a solution containing 30% (w / v) NIPAM,...
Embodiment 3
[0042] Example 3 Preparation of NIPAM / HEMA drug controlled release microsphere carrier:
[0043] 1. Preparation of ordered colloidal crystal microspheres: similar to Example 1.
[0044] 2. NIPAM / HEMA hydrogel filling: the prepared SiO 2 The colloidal crystal microspheres were subjected to hydrophilic treatment, soaked in a mixture of 70% concentrated sulfuric acid and 30% hydrogen peroxide for 12 hours to make the surface of the microspheres hydroxylated. After repeatedly washing with ultrapure water and blowing dry with nitrogen, the colloidal crystal microspheres can be soaked in a solution containing 30% (w / v) NIPAM, 5% (w / v) HEMA, 1 (v / v)% photoinitiated In the hydrogel polymerization precursor solution of the agent, the colloidal solution can be gelled after the microspheres change from white to colored and irradiated with ultraviolet rays for 1-2 minutes. Then the hydrogel microspheres are soaked in deionized water. Due to the different expansion coefficients inside ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com