Method for efficiently expressing Bombyx mori recombinant antibacterial peptides by using lactose culture medium
A silkworm antibacterial peptide, high-efficiency expression technology, applied in the biological field, can solve the problems of high price, low recombinant protein and polypeptide content, low recombinant protein expression yield, etc., and achieves high bacterial density, reduced toxicity, and high protein yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] Further illustrate the implementation process and beneficial technical effects of the present invention with examples below.
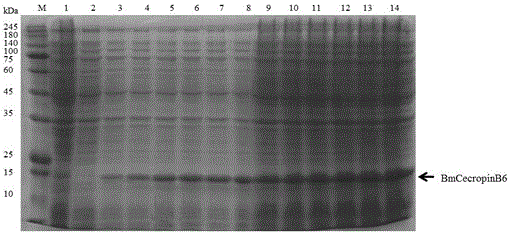
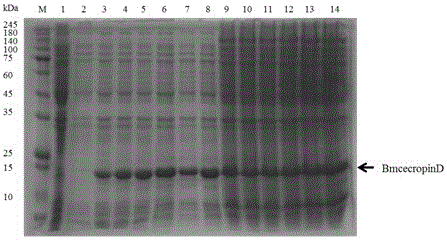
[0030] In order to achieve the above object, the steps of the technical solution adopted in the present invention are as follows: the method for prokaryotic expression of antimicrobial peptides BmcecropinB6, BmcecropinD and Bmmoricin by Escherichia coli expression system mainly includes:
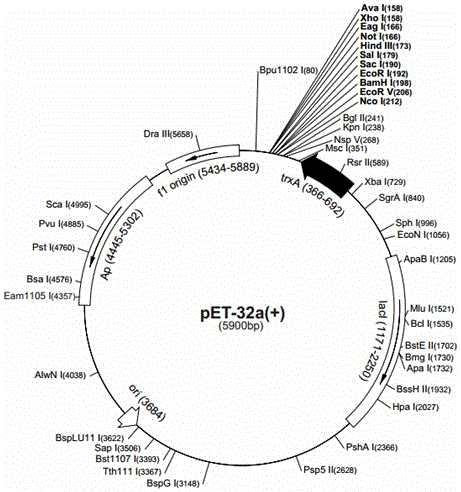
[0031] (1) silkworm BmcecropinB6, BmcecropinD, Bmmoricin gene cloning
[0032] ① Take about 1 g of the midgut tissue of the five-instar and seven-day-old silkworm, wash it with DEPC water, transfer the midgut tissue to a prepared mortar, add liquid nitrogen, grind it into powder, and then press 50-100 mg tissue / mL TRIzol Reagent , until the sample was mixed with TRIzol, and after standing at room temperature for 5 min, 1 / 5 volume of chloroform was added. Thoroughly shake and mix, and centrifuge at 12,000 rpm for 15 minutes at 4°C. Carefully transfer the supe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com