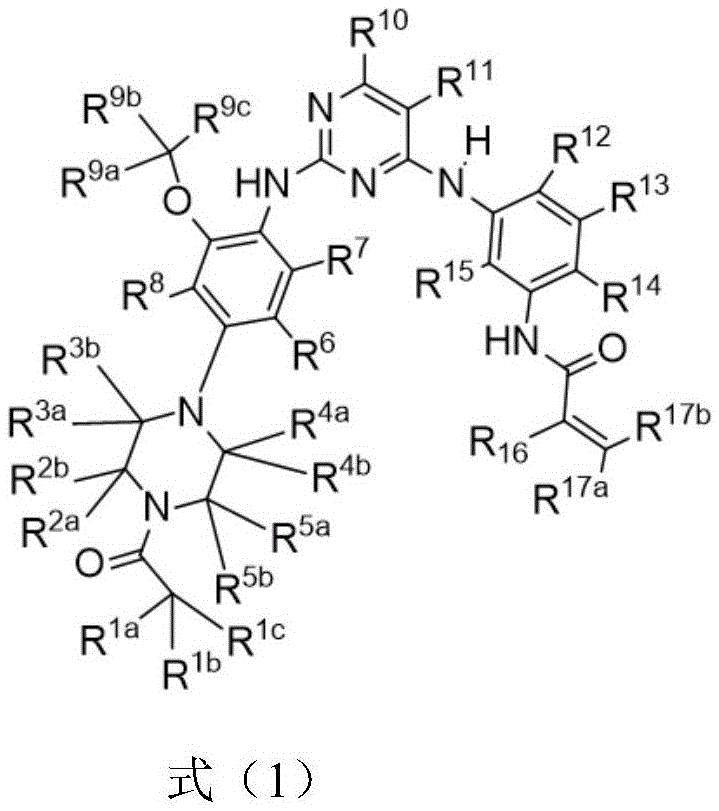
Substituted diaminopyrimidine compound, composition comprising compound and application of compound
A kind of technology of diaminopyrimidine and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] N-(3-(2-(4-acetylpiperazin-1-yl)2-d3-methoxyanilino)-5-trifluoromethylpyrimidinyl-4amino)phenyl was prepared according to the following synthetic route Acrylamide, i.e. compound 11 in the following synthetic route, i.e. the compound of synthetic formula (2);
[0043]
[0044] Take the following steps:
[0045] Step 1: Preparation of 3-(2-chloro-5-trifluoromethyl)pyrimidine-4-aminophenyl tert-butyl ester (compound 3);
[0046] equipped with a magnetic stirrer, N 2 Add n-butanol (9mL) into a 50mL three-necked flask with a bulb and a thermometer, cool in an ice-water bath, keep the internal temperature not exceeding 5°C, add 3-N-Boc-aniline (0.96g, 4.6mmol), slowly add 2,4 -Dichloro-5-trifluoromethylpyrimidine (1.0g, 4.6mmol), then N,N-diisopropylethylamine (0.67g, 5.5mmol) was slowly added dropwise into the reaction solution, and the mixture was iced Stir in a water bath for 1 h, then stir at room temperature for 4 h. A large amount of white solid formed was filtere...
Embodiment 2
[0063] The following synthetic route was used to prepare N-(3-(2-(4-d3-acetylpiperazin-1-yl)2-methoxyanilino)-5-trifluoromethylpyrimidinyl-4 amino)benzene Acrylamide (compound 15), the compound of synthetic formula (3);
[0064]
[0065] Take the following steps:
[0066] Step 1: The synthesis of compounds 12, 13, and 14 was synthesized with reference to compounds 9, 10, and 11 of Example 1, wherein the spectrum of compound 14 is: 1 HNMR(300MHz,DMSO-d6)δ(ppm):10.16(s,1H),8.65(br s,1H),8.29(s,1H),8.08(s,1H),7.76(br s,1H), 7.55-7.48(m,2H),7.27(t,J=8.1Hz,1H),7.17-7.14(m,1H),6.60(d,J=2.7Hz,1H),6.49-6.40(m,1H) ,6.28-6.21(m,2H),5.78-5.74(m,1H),3.77(s,3H),3.44(t,J=4.8Hz,4H),3.00(t,J=4.8Hz,4H), 1.43(s,9H).LC-MS(APCI):m / z=614.2(M+1) + ,purity: 96.1%.
[0067] Step 2: Preparation of N-(3-(2-(4-d3-acetylpiperazin-1-yl)2-methoxyanilino)-5-trifluoromethylpyrimidinyl-4amino)phenyl Acrylamide (compound 15)
[0068] Add tert-butyl-4-(4-(4-(3-acrylamidophenylamino)-5-trifluoromethylp...
Embodiment 3
[0071] The following synthetic route was used to prepare N-(3-(2-(4-acetyl-d8-piperazin-1-yl)2-methoxyanilino)-5-trifluoromethylpyrimidinyl-4-amino) Phenylacrylamide (compound 19), the compound of synthetic formula (4);
[0072]
[0073] According to the method described in Example 1, the difference is that this example uses N-acetyl-d8-piperazine instead of N-acetylpiperazine, thereby obtaining the target compound 19, 1 HNMR(300MHz,DMSO-D6)δ(ppm):10.52(s,1H),9.79(br s,1H),9.33(br s,1H),8.51(br s,1H),7.85(s,1H) ,7.62(d,J=8.1Hz,1H),7.45-7.42(m,1H),7.36(t,J=8.1Hz,1H),7.13-7.10(m,1H),6.77(s,1H), 6.58-6.49(m,1H),6.47-6.22(m,2H),5.78-5.74(m,1H),3.80(s,3H),2.06(s,3H).LC-MS(APCI):m / z=564.2(M+1) + ,purity: 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com