Preparation method of light alkane isomerization catalyst and isomerization method of light alkane
A technology of isomerization and catalyst, which is applied in the preparation of light alkane isomerization catalyst and the field of isomerization of light alkane, can solve the problem of low isomerization yield, achieve catalyst stability, improve stability, Effect of improving isomerization performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] About the preparation method of light alkane isomerization catalyst
[0039] The mass dosage ratio of Zr-containing salt to Fe-containing salt:
[0040] In the present invention, the mass dosage ratio of the Zr-containing salt to the Fe-containing salt is not particularly limited, usually in step a, the Fe-containing salt is further added, and the mass dosage ratio of the Zr-containing salt to the Fe-containing salt is 1.1:1~10:1.
[0041] If the mass ratio of the Zr-containing salt to the Fe-containing salt is less than 1.1:1, the H 2 SO 4 The load is too low, the acidity is too weak, the isomerization reaction activity is reduced, and the mass dosage ratio of Zr-containing salt to Fe-containing salt is greater than 10:1, because the amount of Zr-containing salt is too much, resulting in waste, and the load h 2 SO 4 Reduced acid strength, reduced isomerization reactivity, and no other beneficial effects.
[0042] Fe-containing salts:
[0043] In the present i...
Embodiment 1
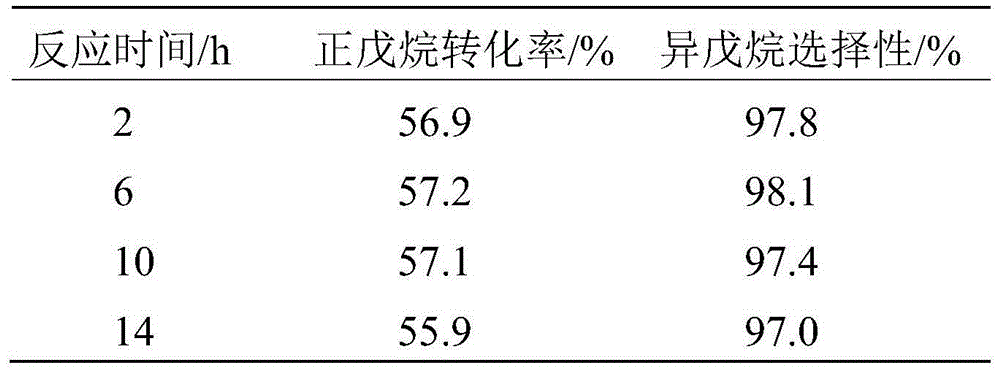
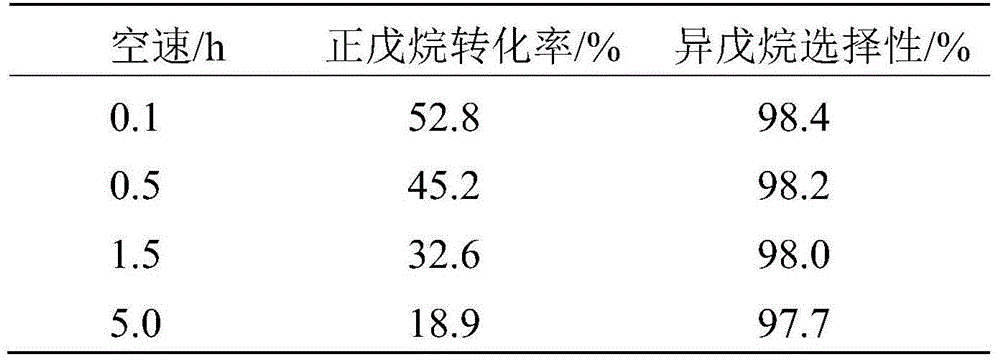
[0063] Embodiment 1 prepares catalyst
[0064] Prepare two solutions separately, the first solution is 96.6g ZrOCl 2 ·8H 2 O was dissolved in 150mL of deionized water to prepare a solution with a Zr content of 2mol / L; the second solution was to mix 27.1g (NH 4 ) 6 h 2 W 12 o 40 Dissolve in 100mL of deionized water to prepare a solution with a W content of 1.1mol / L, and then add 52g of ammonia water with a concentration of 1.5mol / L. After the first solution is prepared, slowly add the second solution dropwise to the first solution under rapid mechanical stirring, and it takes 30-45 minutes to complete the dropwise addition. Then the solution was adjusted to pH=9 with ammonia water, and then the stirring was continued for 1.5-2 hours. The resulting precipitate was filtered and washed 4 times with deionized water. Then the washed product was dried in an oven at 110° C. for 12 hours. The solid obtained after drying was separated by SO 4 2- A standard with a loading of 2...
Embodiment 2
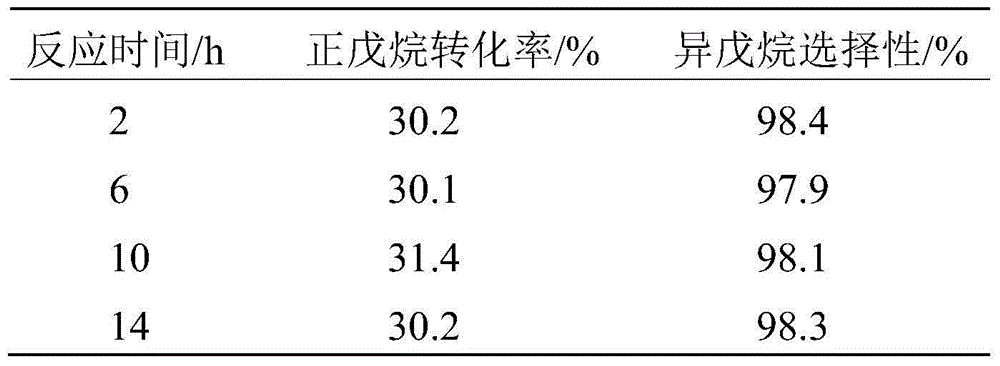
[0065] Embodiment 2 prepares catalyst
[0066] Prepare two solutions separately, the first solution is 24.1g ZrOCl 2 ·8H 2 O was dissolved in 150mL of deionized water to prepare a solution with a Zr content of 0.5mol / L; the second solution was to mix 49.3g (NH 4 ) 6 h 2 W 12 o 40 Dissolve in 100mL of deionized water to prepare a solution with a W content of 2mol / L, and then add 30g of ammonia water with a concentration of 1.5mol / L. After the first solution is prepared, slowly add the second solution dropwise to the first solution under rapid mechanical stirring, and it takes 30-45 minutes to complete the dropwise addition. Then the solution was adjusted to pH=9 with ammonia water, and then the stirring was continued for 1.5-2 hours. The resulting precipitate was filtered and washed 4 times with deionized water. Then the washed product was dried in an oven at 130° C. for 8 hours. The solid obtained after drying was separated by SO 4 2- A standard with a loading of 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com