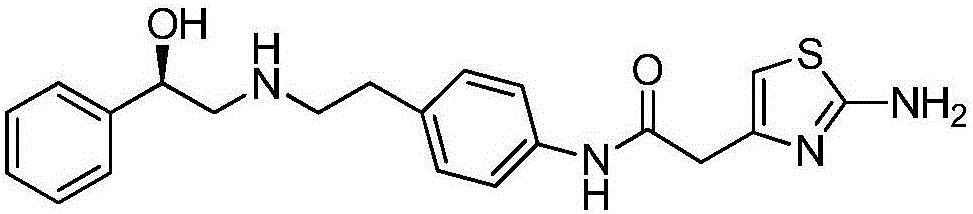
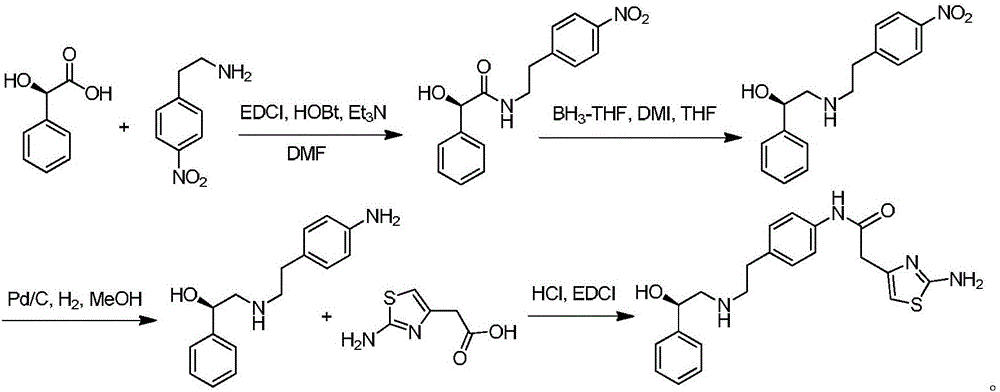
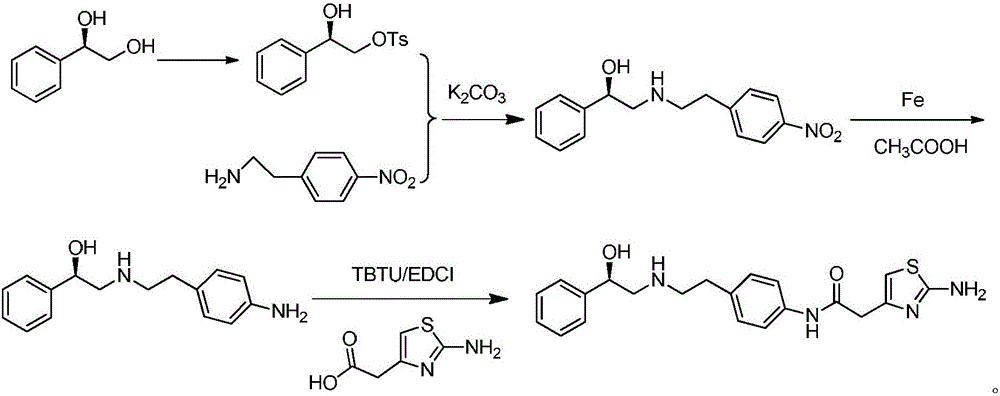
Efficient synthesis method of mirabegron
A synthetic method and high-efficiency technology, applied in organic chemistry and other fields, can solve the problems of high equipment requirements and expensive equipment, and achieve the effects of cheap raw materials, reduced reaction costs, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] In a 500mL reaction flask, dissolve 32g of p-aminophenylacetonitrile (0.2mol) in 200mL of methanol, then add 3.2g of palladium-calcium catalyst, slowly add 32g of hydrazine hydrate dropwise under nitrogen protection, and react at 40°C for 12h after the addition , TLC monitoring the reaction of the raw materials is complete, filter the reaction solution, rotary evaporation to remove the solvent methanol, add a certain amount of dichloromethane, wash with water three times, the organic phase is spin-dried to obtain 30g of p-aminophenethylamine. 1 H NMR (400MHz, DMSO-d 6 )δ6.98(dt,J 1 =8.4Hz,J 2 =2.0Hz,2H,Ar-H),6.63(dt,J 1 =8.4Hz,J 2 =2.0Hz,2H,Ar-H),3.57(brs,2H,NH 2 -H),2.89(t,J=6.8Hz,2H,CH 2 -H),2.63(t,J=6.8Hz,2H,CH 2 -H),1.15(bra,2H,NH 2 -H).
Embodiment 2
[0033]
[0034]In a 500mL reaction flask, add 20g (R)-1-phenyl-1,2-ethanediol (0.145mol) and 25g piperidine (0.29mol) into 200mL dichloromethane, and slowly Add 100 mL of dichloromethane solution dissolved with 18 g of methanesulfonyl chloride (0.16 mol), rise to room temperature and react for 1 h after the dropwise addition, TLC monitors that the raw materials have reacted completely, add a certain amount of water to wash, separate the organic phase, and wash the water phase with two Extracted with methyl chloride, the combined organic phases were spin-dried to obtain 21 g of (R)-1-phenyl-1-hydroxy-2-methanesulfonate-ethane. 1 H NMR (400MHz, CDCl 3 ): δ7.77(d,J=8.2Hz,2H,Ar-H),7.36-7.27(m,3H,Ar-H),4.97(dd,J 1 =8.6Hz,J 2 =3.3Hz,1H,CH-H),4.15(dd,J 1 =10.3Hz,J 2 =3.3Hz,1H,CH 2 -H),4.05(dd,J 1 =10.3Hz,J 2 =8.6Hz,1H,CH 2 -H),2.45(s,3H,CH 3 -H).
Embodiment 3
[0036]
[0037] In a reaction flask equipped with a water separator, add 32.5 g (0.25 mol) of ethyl acetoacetate into 300 mL of cyclohexane, then add 20 g (0.275 mol) of pyrrolidine and 0.5 g of p-toluenesulfonic acid monohydrate ( 2.63mmol), warming up to reflux and removing water, cooling to room temperature after reacting for 5h, filtering the reaction solution, steaming out cyclohexane and pyrrolidine in the filtrate, adding the resulting distilled product to 500mL of anhydrous pyridine, and then Add 16g (0.5mol) of elemental sulfur, set the reaction temperature at 0°C, slowly add 50mL of pyridine solution with 21g (0.5mol) of cyanamide dissolved in it dropwise, after the dropwise addition, slowly raise the temperature to 90°C for 60 minutes, and monitor the reaction of raw materials by TLC Completely, cooling to room temperature has a large amount of solid to separate out, suction filtration reaction liquid, filter cake is dried after washing with ether (200mL * 3), obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com