Intraocular inflammation resisting implant as well as preparation method and application thereof
An implant and endophthalmitis technology are applied in the field of implants for the treatment of intraocular inflammation and their preparation to achieve the effects of controlling inflammatory symptoms and reducing systemic drug toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation method
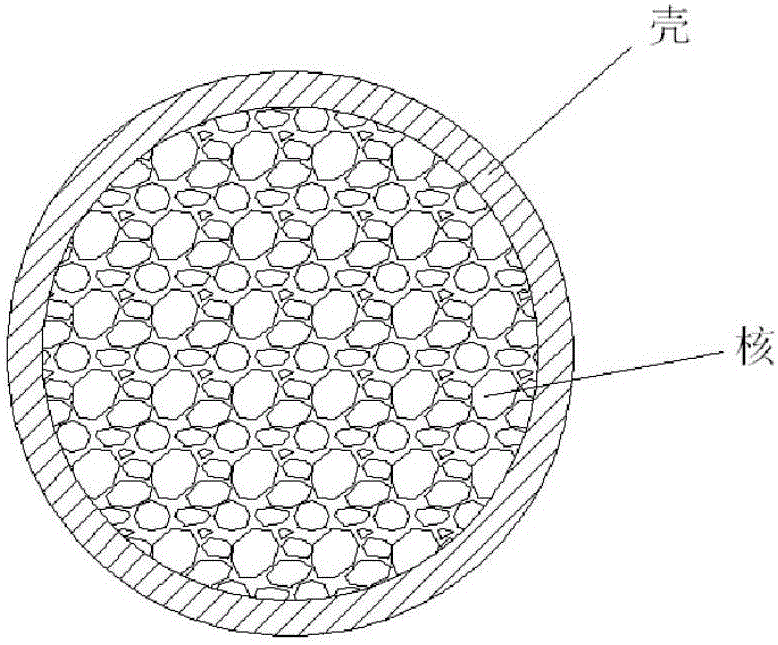
[0023] Under the protection of high-purity nitrogen, 1.0g of chlorogenic acid and 9.0g of lactide-co-glycolide (weight average molecular weight: 100,000) were mixed evenly, in a miniature twin-cone twin-screw extruder, at 180°C , co-melt-extruded columnar body, as the "core".
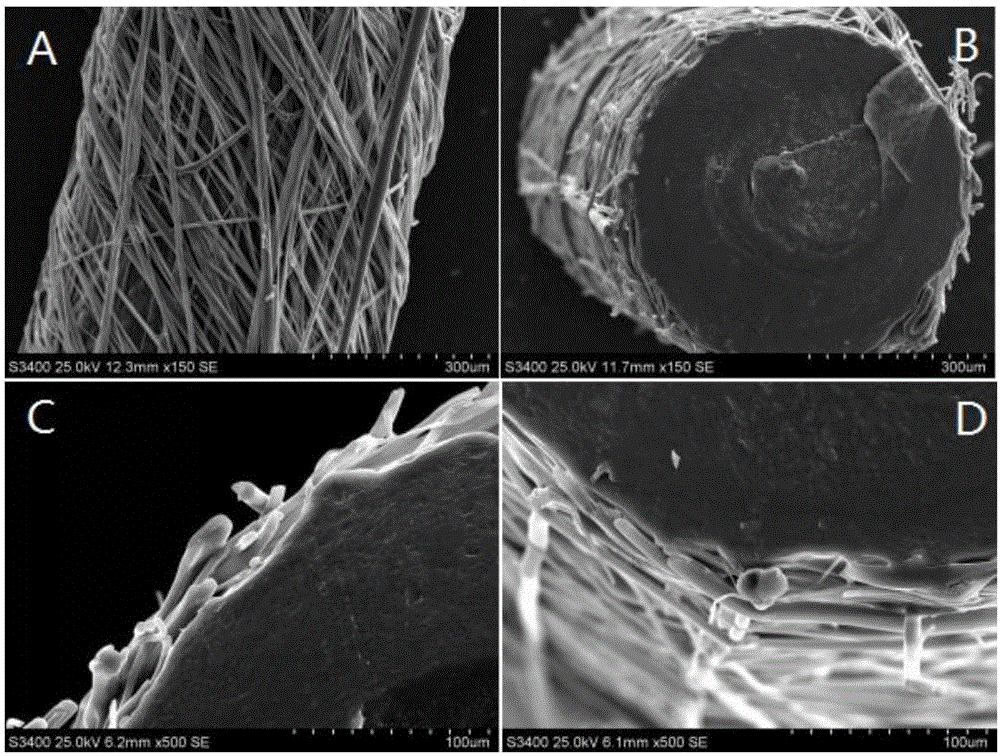
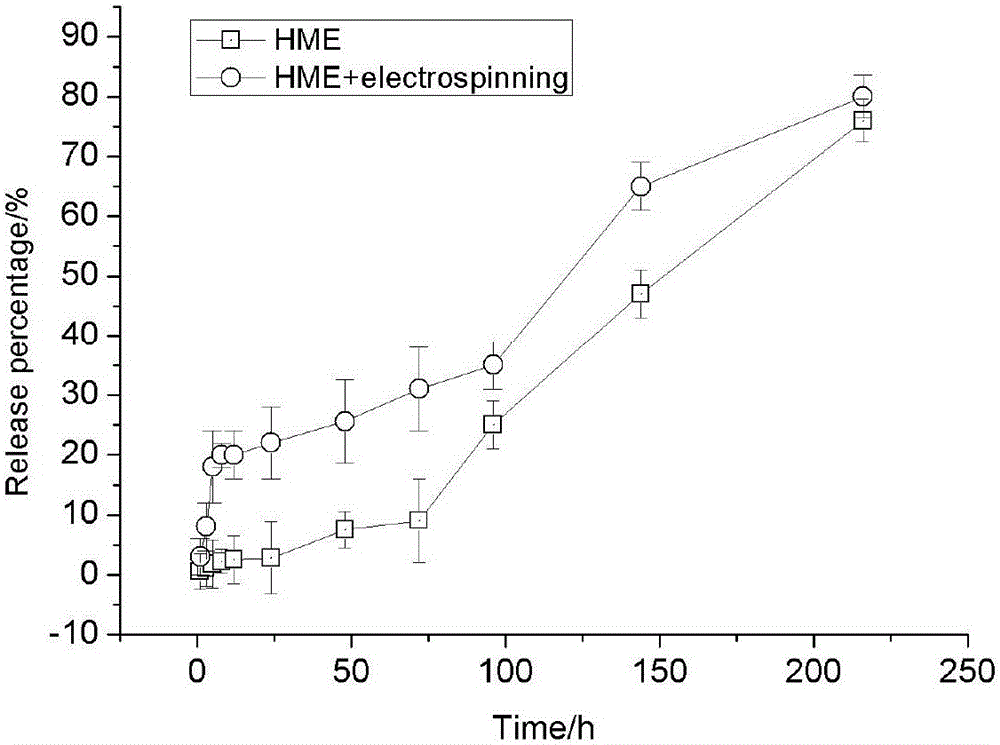
[0024] 2.0g lactide-glycolide copolymer (lactide accounts for 50% by mole percent, intrinsic viscosity is 0.7) and 0.04g chlorogenic acid are dissolved in 20mL " chloroform-acetone " mixed solvent (V 氯仿 :V 丙酮 =2:1), the prepared mixed solution is passed through an electrospinning machine, and a layer of electrospinning film loaded with chlorogenic acid is wrapped around the columnar body as a "shell". The process parameters of electrospinning were: voltage 30kV; injection rate: 2.0mL / h; distance between nozzle and receiving plate: 150mm; electrospinning time: 10h, and the implant was prepared.
[0025] The prepared implant was cooled and then cut to obtain a cylindrica...
Embodiment 2
[0034] Under the protection of high-purity nitrogen, mix 0.1g of chlorogenic acid and 9.9g of polyglycolide (weight average molecular weight: 10,000) evenly, in a miniature twin-cone twin-screw extruder (SJZS-7AY), at the extrusion temperature At 130°C, they were co-melted and extruded to form a columnar body as a "core".
[0035]Dissolve 2g of polylactide and 0.08g of chlorogenic acid in 20mL of "chloroform-acetone" mixed solvent (V 氯仿 :V 丙酮 =2:1)), the prepared mixed solution is passed through an electrospinning machine, and a layer of electrospinning film loaded with chlorogenic acid is wrapped around the columnar body as a "shell". The process parameters of electrospinning were: voltage 30kV; injection rate: 2.0mL / h; distance between nozzle and receiving plate: 300mm; electrospinning time: 15h, and the implant was prepared.
[0036] The prepared implant was cooled and then cut to obtain a cylindrical shape with a diameter of 2.0 mm and a length of 5.0 mm to obtain an ant...
Embodiment 3
[0038] Under the protection of high-purity nitrogen, 0.5g chlorogenic acid, 4.5g polylactide (weight-average molecular weight: 5,0000), and 5.0g poly-dioxanone (weight-average molecular weight: 50,000) were mixed evenly, and In a micro-twin-cone twin-screw extruder (SJZS-7AY), at an extrusion temperature of 150°C, they are co-melted and extruded to form a columnar body as a "nucleus".
[0039] Dissolve 2g polyglycolide and 0.10g chlorogenic acid in 20mL "chloroform-acetone" mixed solvent (V 氯仿 :V 丙酮 =1:1), the prepared mixed solution was passed through an electrospinning machine, and a layer of electrospinning film loaded with chlorogenic acid was wrapped around the columnar body as a "shell". The process parameters are: voltage 30kV; injection rate: 2.0mL / h; distance between the nozzle and the receiving plate: 200mm; electrospinning time: 8h, and the membrane is obtained.
[0040] The prepared implant was cooled and then cut to obtain a diameter of 2.0 mm and a length of 5....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com