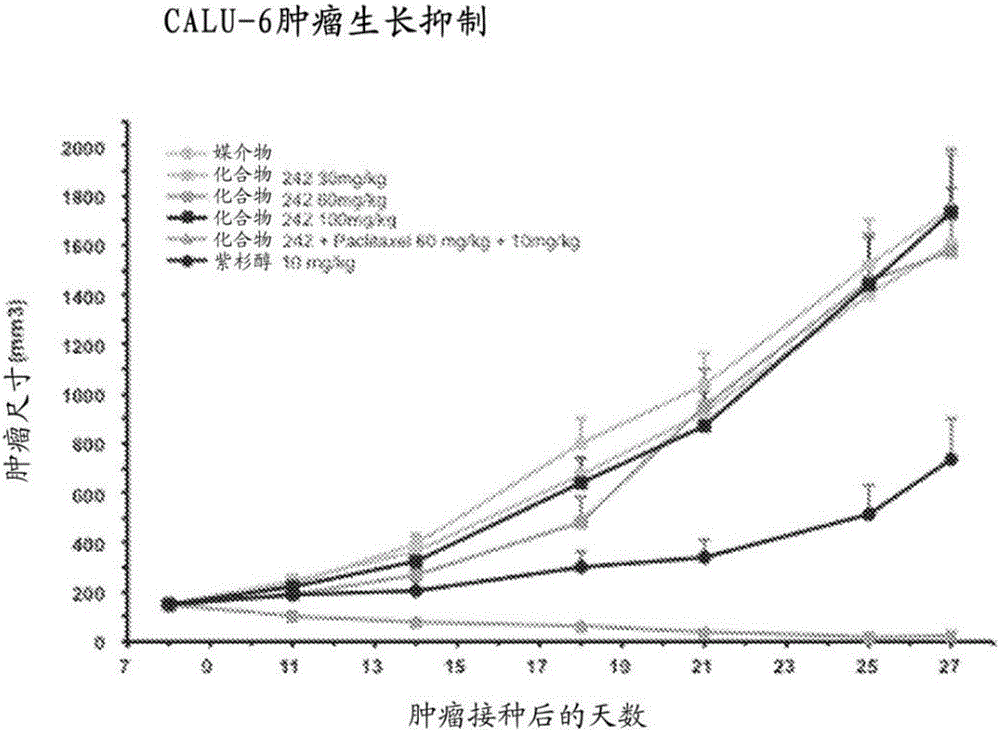
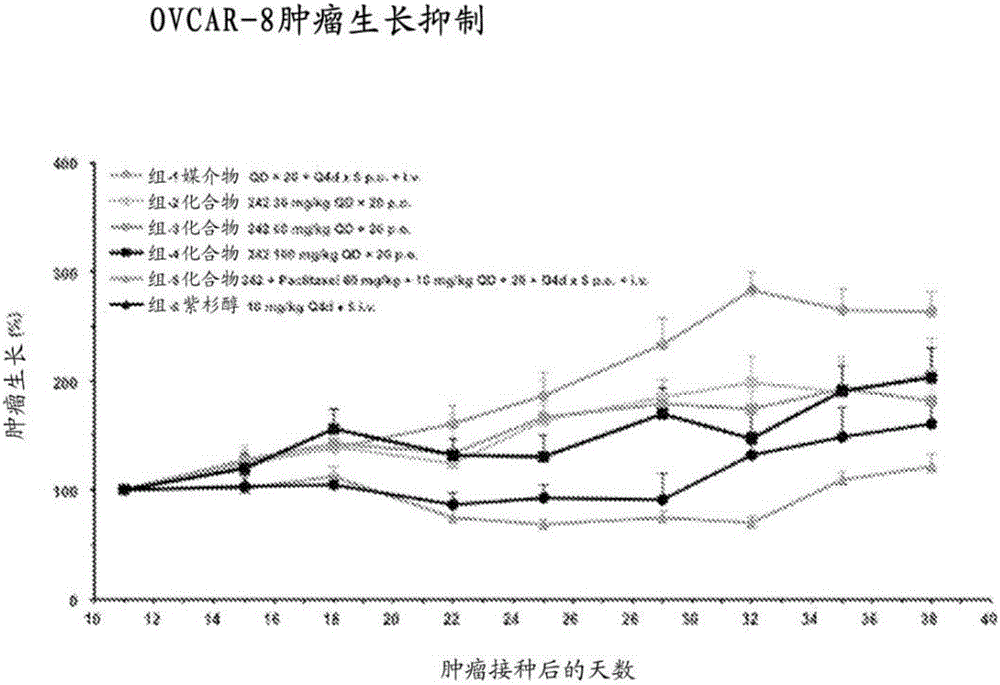
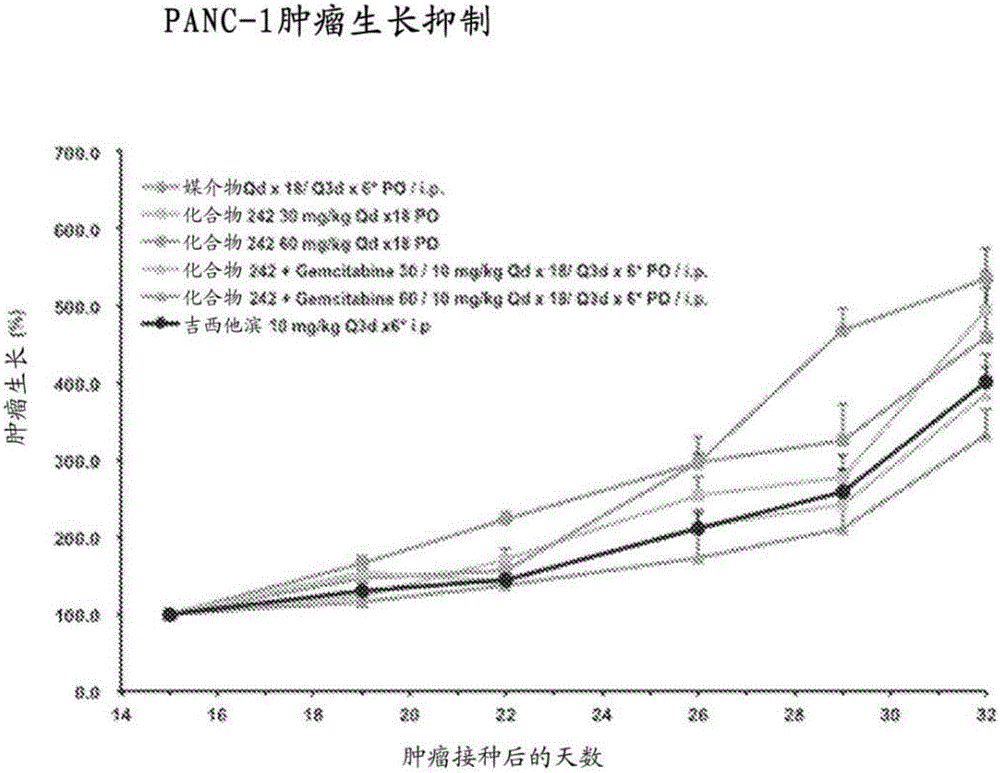
Heterocyclic modulators of lipid synthesis and combinations thereof
A technology of fatty acid synthase and compounds, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, medical preparations containing active ingredients, etc., can solve problems such as anemia, impaired nutrient absorption, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0678] Synthesis of Compounds of the Disclosure
[0679] General: All reactions and manipulations described were performed in a well-ventilated fume hood. Manipulations and reactions under high or reduced pressure are performed behind explosion-proof shields. Abbreviations: ACN: acetonitrile; AcOH: acetic acid; AIBN: azobisisobutyronitrile; BF 3 -Et 2 O: boron trifluoride diethyl ether; (Boc) 2 O: di-tert-butyl dicarbonate; BuLi: butyllithium; CDI: 1,1'-carbonyldiimidazole; DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene; DCE: 1,2-dichloroethane; DCM: dichloromethane or methylene chloride; DIEA: N,N-diisopropylethylamine; DMA: N,N-dimethylacetamide ; DMAP: 4-dimethylaminopyridine; DME: 1,2-dimethoxyethane; DMEDA: N, N'-dimethylethylenediamine; DMF: N, N-dimethylformamide; DMSO: dimethylsulfoxide; DPPP: 1,3-bis(diphenylphosphino)propane; EDC: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; EDCI: 1- Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride; EtOAc: ethyl acetate;...
Embodiment 2
[2099] Activity of Compounds of the Disclosure
[2100] antiviral activity
[2101] The antiviral activity of the compounds was assessed using the HCV1b replicon system. The replicon was constructed using the ET (luc-ubi-neo / ET) cell line, a Huh7 human with a stable luciferase (Luc) reporter gene and three cell culture-adaptive mutations Liver cancer cell lines (Pietschmann et al. (2002) J. Virol. 76:4008-4021). HCV Replicon Antiviral Evaluation Assay The effect of compounds at ten triplicate dilutions was studied. Subconfluent cultures of ET cell lines were seeded into 96-well plates dedicated to analysis of cell viability (cytotoxicity) or antiviral activity, and the next day, drugs were added to the appropriate wells. After 72 hours, cells were processed while still subconfluent. Determination of EC 50 Value (concentration that suppresses 50% of HCV RNA replicons), CC 50 value (concentration that reduces cell viability by 50%) and SI value (selection index: CC 50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com