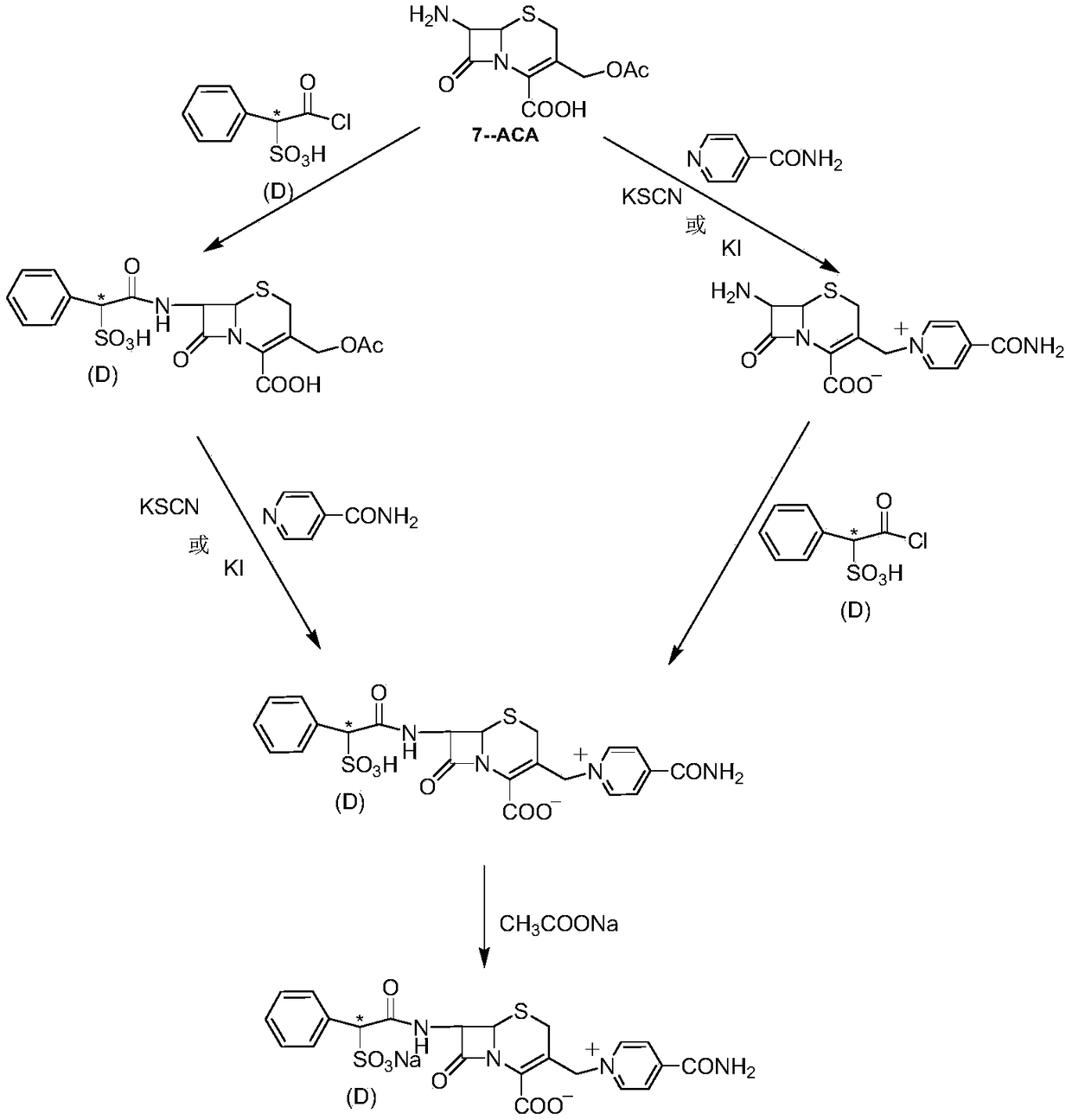
Medicinal cefsulodin sodium compound and preparation method thereof
A technology of cefsulodin sodium and its compounds, applied in the field of medicine, can solve problems such as insufficient safety, high cost, and low yield, and achieve the effects of high safety, low equipment requirements, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
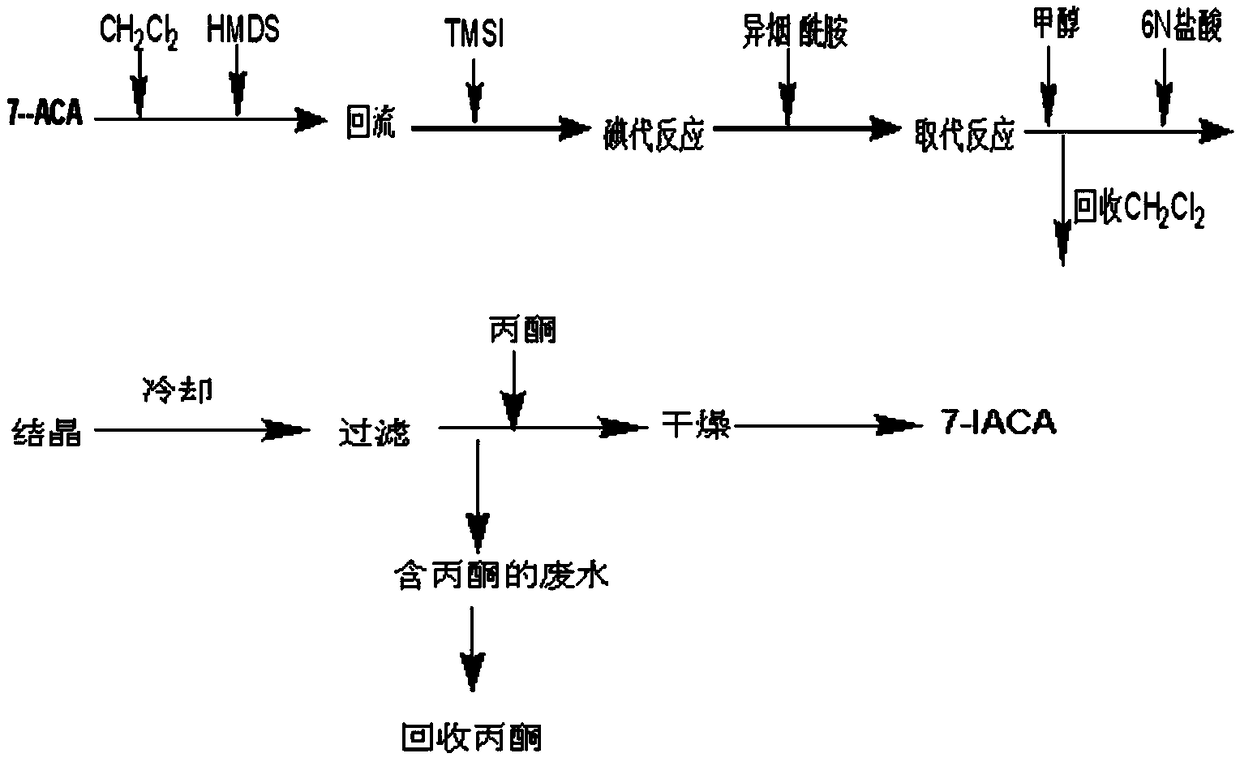
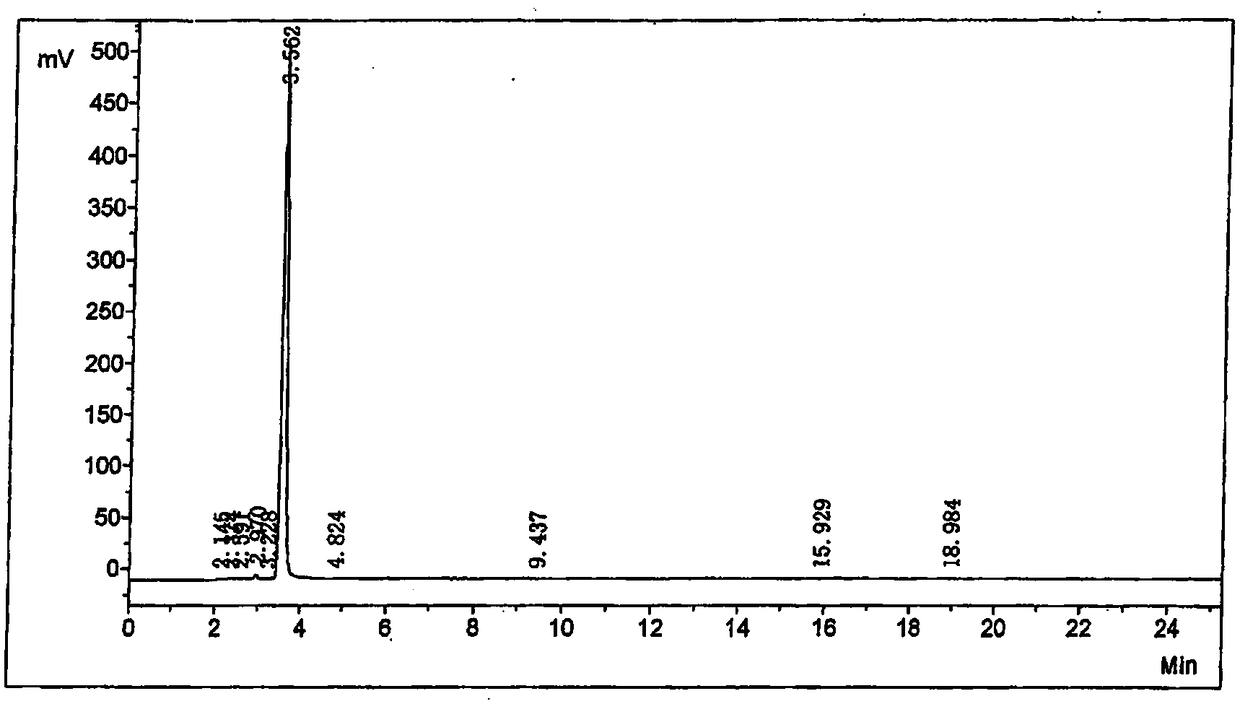
[0043] like figure 2 As shown, add 8.0kg 7-ACA, 86.7kg dichloromethane, 14.5kg hexamethyldisilamine (HMDS) and 29ml concentrated sulfuric acid in a 200-liter reaction tank as a starter, and reflux the reaction tank after 4 hours. Cool down the temperature, maintain at -5 ~ 0 ℃, add 9.38kg trimethylsilyl iodide (TMS-I), stir and react for 3 hours, add 3.74kg isonicotinamide, slowly raise the temperature, reflux for 2 hours, and then react Cool the solution to 0°C, add 9.42kg of methanol, stir for 0.5 hours, add 35.2L of 6N hydrochloric acid, heat up to 20°C, stir for 0.5 hours, then extract the organic phase with 10kg of water, combine the water phases, transfer to a 150L reaction tank, and cool down to 0~5℃, add 6L 6.6% hydrogen peroxide aqueous solution, stir for 10 minutes after addition, filter, wash the filtered residue with 5kg water, combine the filtrate, add 3kg activated carbon and stir for decolorization for 1 hour, filter, wash with 5kg water The filter layer was c...
Embodiment 2
[0046] In above-mentioned embodiment 1, adopt 1.2-dichloromethane, dichloroethane, acetonitrile, N-N dimethylformamide, N-N dimethylacetamide, dimethyl sulfoxide, ethyl acetate, methyl acetate and acetic acid One or more of the butyl esters can replace dichloromethane as the organic solvent, and the target product 7-IACA can be prepared with the same technical characteristics, and the purity is 98.87%.
Embodiment 3
[0048] In the above-mentioned embodiment 1, one or more of trimethylchlorosilane, trimethyl iodosilane, N, O-bistrimethylsilylacetamide and bistrimethylsilylurea are used to replace hexamethyldimethoxysilane Silamine (HMDS) is used as a silicon protection reagent, and the target product 7-IACA can be prepared by adopting other identical technical features, with a purity of 98.87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com