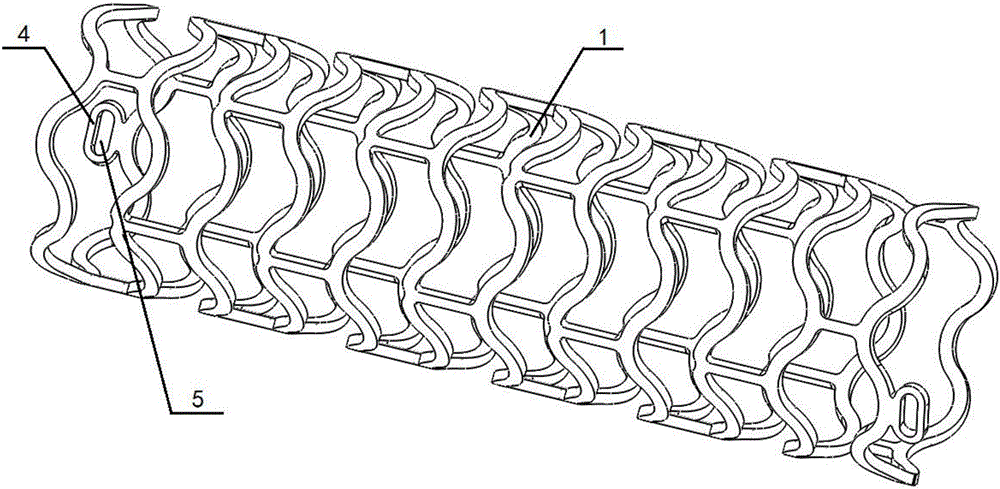
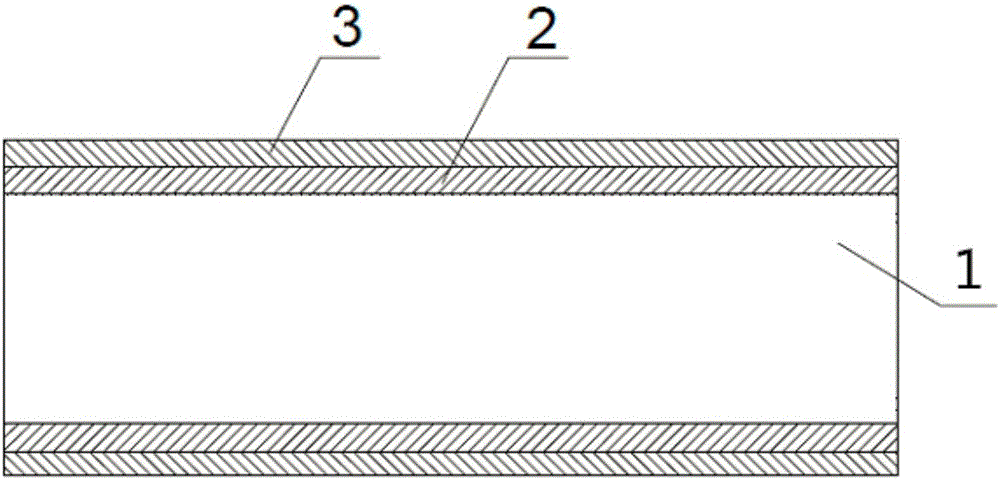
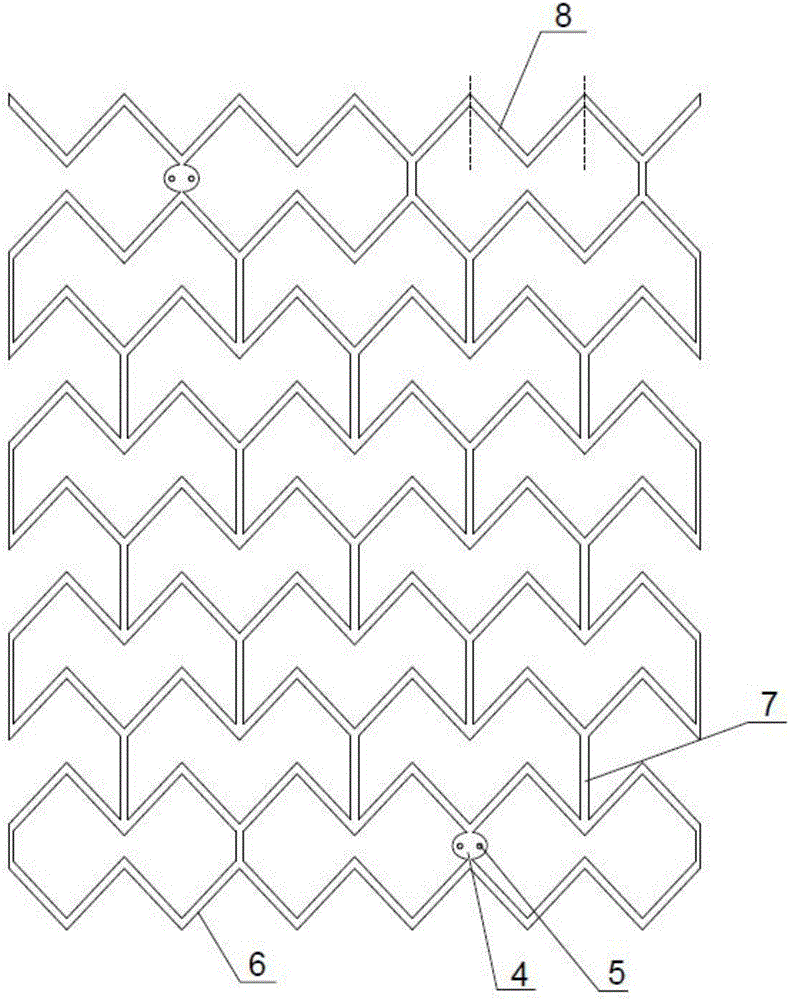
Full-degradable vascular stent for vascular disease treatment and production method of full-degradable vascular stent
A technology for vascular stents and vascular diseases, which is applied in the field of fully degradable vascular stents and its preparation, can solve problems such as hindering the recovery of vascular functions, and achieve the effects of convenient operation and use, stable performance, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation method of the fully degradable vascular stent for the treatment of vascular diseases, comprising the following steps:
[0067] 1) Raw material preparation:
[0068] Taking polylactic acid as raw material A, the number-average molar molecular weight of polylactic acid is 100,000;
[0069] 2) Processed into pipes:
[0070] First, heat the raw material A to a molten state, and the heating temperature is 200 ° C. Fill the molten material into the pipe forming mold, and then naturally cool until the internal temperature of the material drops to room temperature, that is, it is processed into a cylindrical pipe; the processed cylinder Profile pipe, its outer diameter is 2mm, and the thickness of the pipe wall is 0.5mm;
[0071] 3) Pipe reinforcement and toughening:
[0072] First place the cylindrical pipe in a constant temperature oven at 60°C for 10 hours to promote the orderly formation of the crystal nucleus of the original structure of the material to ...
Embodiment 2
[0084] The preparation method of the fully degradable vascular stent for the treatment of vascular diseases, comprising the following steps:
[0085] 1) Raw material preparation:
[0086] Put the polylactic acid and polylactic acid-trimethylene carbonate copolymer into the twin-screw extruder in a ratio of 70:30 in mass ratio for blending and granulation to obtain raw material B; the twin-screw extruder blending and pelletizing temperature is 180℃, the speed is 20rpm;
[0087] Wherein, the number-average molar molecular weight of polylactic acid is 400,000, and the number-average molar molecular weight of polylactic acid-trimethylene carbonate copolymer is 50,000;
[0088] 2) Processed into pipes:
[0089] The raw material B is placed in a plastic extruder, and the raw material is heated to a molten state in the plastic extruder, and its heating temperature is 210 ° C, then the melt is extruded from the pipe forming die, and finally cooled naturally until the material is Wh...
Embodiment 3
[0103] The preparation method of the fully degradable vascular stent for the treatment of vascular diseases, comprising the following steps:
[0104] 1) Raw material preparation:
[0105] Taking polylactic acid as raw material A, the number-average molar molecular weight of polylactic acid is 100,000;
[0106] 2) Processed into pipes:
[0107] The raw material A and the polylactic acid-trimethylene carbonate copolymer are placed in two extrusion heads in the extruder, respectively, and the two substances are heated to a molten state in the two extrusion heads, respectively. The heating temperature is 180°C; the extruded pipe has two layers, the material of the inner layer is polylactic acid, and the material of the outer layer is polylactic acid-trimethylene carbonate copolymer; finally cooled naturally until the internal temperature of the material drops to room temperature, That is, it is processed into a cylindrical pipe; the outer diameter of the processed cylindrical pi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com