Anti-inflammatory activity of a paeonol homologue and its preparation application
A homologue, paeonol technology, applied in the field of inflammation treatment products, can solve the problem of low anti-inflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
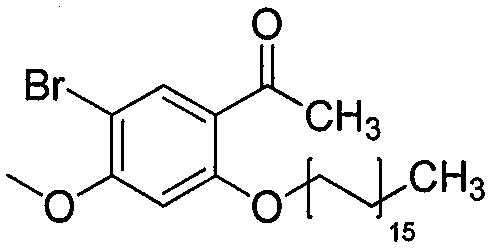
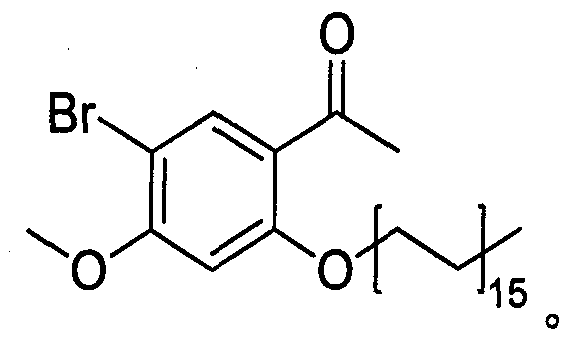
[0018] The synthesis of medicine (Al) described in embodiment 1
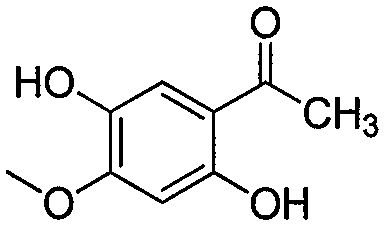
[0019] Weigh 7g of paeonol, 120ml of chloroform, anhydrous AlCl 3 Add 0.7g into a three-necked flask, stir in an ice-water bath, and then slowly add 3ml of bromine to the reaction. After reacting for 2 hours, TLC silica gel plate was used to track at the same time. When new spots were formed and the color of the new spots was under a 254nm ultraviolet lamp, the color did not change, that is, the reaction was stopped. The reaction solution was suspended and evaporated, and recrystallized several times with acetone to obtain white crystals. Accurately weighed to obtain 0.8g of white crystals, K 2 CO 3 Add 0.8g of powder, 0.005mol of brominated alkanes, 10ml of DMF, and 10ml of acetone into a 150ml three-necked bottle, and reflux at 60°C for 2 hours. The reaction solution was filtered to remove the filter residue. Rotary evaporation filtrate, it is carried out rotary evaporation to dryness, after adding a cert...
Embodiment 2
[0021] The influence of medicine (Al) described in embodiment 2 on the mouse paw swelling degree induced by complete Freund's adjuvant
[0022] Preparation of Kunming mouse animal model and experiment of diosmin on mouse paw swelling induced by complete Freund's adjuvant. 50 Kunming mice, half male and half female, body weight 18-22g, were randomly divided into 5 groups: blank group, model group, celecoxib group (positive control), Al low-dose group, Al high-dose group, each group 10 mice. According to the modeling method of Liu Mingpei et al., the blank group was intradermally injected with 0.1mL normal saline at the plantar part of the right hind foot of each mouse, and the other groups were injected with complete Freund's adjuvant, and on the 7th day after the first injection of Freund's adjuvant, The same dose of Freund's adjuvant was injected once every day. On the seventh day, redness and swelling of toes and even bare joints appeared in the inflamed parts, and the inf...
Embodiment 3
[0027] Effects of the drug (Al) described in Example 3 on lipid mediators in serum of mice with adjuvant-induced paw swelling.
[0028] 3.1 Determination of cyclooxygenase 2 (COX-2)
[0029] According to the instructions of the mouse cyclooxygenase 2 ELISA kit, the content of COX-2 was determined. Take out the required strips from the aluminum foil bag after equilibrating at room temperature for 20 minutes, set the standard wells and sample wells, add 50 μl of standard substances of different concentrations to each of the standard wells; first add 10 μl of the sample to be tested, and then add 10 μl of sample diluent ; Blank wells are not added. In addition to the blank wells, add 100 μl of HRP-labeled detection antibody to the standard wells and sample wells, seal the reaction wells with plate sealing film, and incubate for 60 min in a 37°C water bath or incubator. Discard the liquid, pat dry on absorbent paper, fill each well with washing solution, let it stand for 1 min, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com