A kind of lithium battery electrolyte and the obtained lithium primary battery
An electrolyte and lithium battery technology, applied in the field of lithium primary batteries, can solve problems such as battery failure to work normally, shorten battery life, destroy passivation films, etc., to improve electrochemical performance, prevent graphite layer peeling, and improve safety performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
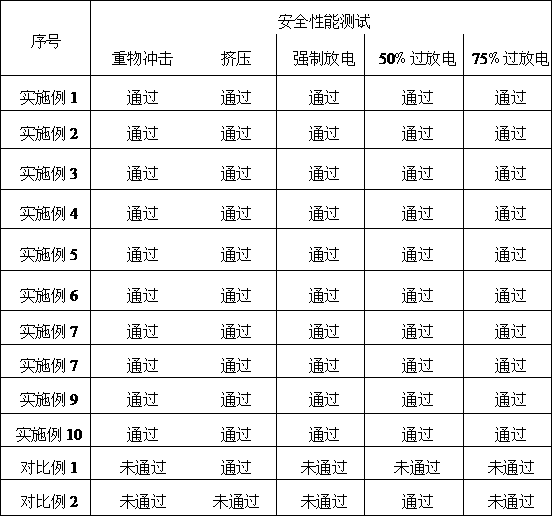
Examples
Embodiment 1
[0015] The positive active material is selected from manganese dioxide, conductive agent carbide, and binder. Pure water is added and stirred until uniform. The dispersed positive active material slurry is coated on the positive electrode aluminum foil current collector. After rolling, it is cut into the positive electrode of the battery. piece. After the positive electrode sheet is dried and completely removed the moisture, it is placed in an environment with a relative humidity lower than 1.0%, and a porous separator is inserted between the negative electrode (metal aluminum or lithium aluminum alloy) and the positive electrode sheet to separate the positive and negative electrodes. The film is wound into a cylindrical cell, and the wound cell is placed in the battery shell (the shell is a steel shell, aluminum shell or aluminum-plastic film), and the battery is assembled by liquid injection.
[0016] In the electrolyte part, use xLiFSI-yLiClO 4 As a mixed lithium salt (45%...
Embodiment 2
[0018] In the same way as in Example 1, the positive electrode material is carbon monofluoride, and xLiFSI-yLiClO is used. 4 As mixed lithium salt (45%e ―PC f As an anti-corrosion agent (wherein the concentration of lithium dioxalate borate LiBOB in the anti-corrosion agent is 1mol / L, e , f is the solvent mass percentage, of which 10%e f 2 ―EC is added to the electrolyte as a composite flame retardant. Among them, the total lithium ion concentration of the mixed lithium salt is 1 mol / L, and in the mixed lithium salt, the mass percentage of LiFSI is 50%, and the LiClO 4 The mass percentage is 46%, the lithium salt LiBOB-AN in the anti-corrosion agent 10% ―PC 90% accounting for the mixed lithium salt LiFSI-LiClO 4 -4% of the total mass percentage of LiBOB. In the composite flame retardant, the phosphorus-containing flame retardant triethyl phosphate TEP accounts for 3% of the total weight of the electrolyte, and the fluorine-containing flame retardant CHF 2 ―EC accounts f...
Embodiment 3
[0020] In the same manner as in Example 1, iron disulfide was used as the positive electrode material, and xLiFSI-yLiClO was used. 4 As mixed lithium salt (45%e ―PC f As an anti-corrosion agent (wherein the concentration of lithium dioxalate borate LiBOB in the anti-corrosion agent is 1mol / L, e , f is the solvent mass percentage, of which 10%e f 3 ―EC is added to the electrolyte as a composite flame retardant. Among them, the total lithium ion concentration of the mixed lithium salt is 1.1 mol / L. In the mixed lithium salt, the mass percentage of LiFSI is 55%, and LiClO 4 The mass percentage is 41%, the lithium salt LiBOB-AN in the anti-corrosion agent 10% ―PC 90% accounting for the mixed lithium salt LiFSI-LiClO 4 -4% of the total mass percentage of LiBOB. In the composite flame retardant, the phosphorus-containing flame retardant triphenyl phosphate TPP accounts for 3% of the total weight of the electrolyte, and the fluorine-containing flame retardant CF 3 ―EC accounts...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com