Chemically-modified electrode for caffeine detection and preparation and application thereof
A chemical modification, caffeine technology, applied in the field of food safety monitoring, can solve the problems of narrow linear range and high detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, chemical modification electrode
[0043] (1) Pretreatment of the glassy carbon electrode: carefully polish the glassy carbon electrode to the mirror surface with a polishing cloth and Al2O3 powder, remove the Al2O3 powder adsorbed on the surface by ultrasonic cleaning, and then dilute the Al2O3 powder in 1:1HNO 3 , 1:1 ethanol, double-distilled water to clean the surface of the electrode, and dry it in the air. Then dip the electrode into a 1×10 -3 M K 3 Fe(CN) 6 and 0.1M KCl electrolyte solution for cyclic voltammetry scanning, when the redox peak potential separation is less than 80 mV, it means that the electrode grinding and polishing is qualified.
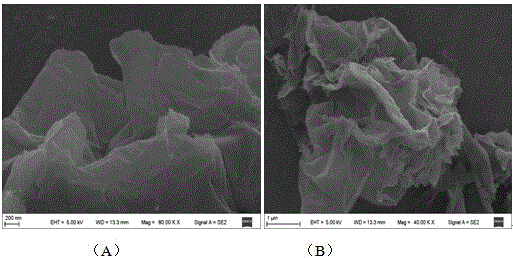
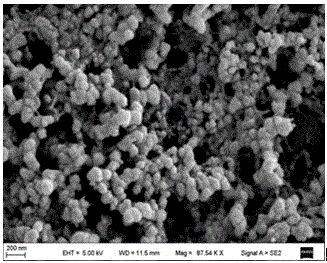
[0044] (2) Preparation of reduced graphene oxide (rGO): (a) First, accurately weigh 2.0 g of graphite powder and place it in a round-bottomed flask, then slowly add 46 mL of concentrated sulfuric acid solution, and stir magnetically in an ice-water bath for 30 min; then place the Slowly add 6.0g of po...
Embodiment 2
[0047] Example 2, using chemically modified electrodes to detect CAF in beverages
[0048] In order to evaluate the practicability of the modified electrode, the prepared modified electrode 3DAuNPs-cys / Nafion-rGO was used to detect CAF in local commercial cola and functional drinks by differential pulse voltammetry. With 0.1M H containing caffeine at pH=2.0 2 SO 4 The buffer solution is an electrolyte, and the scan rate is 90~190 mV, the electrode potential is -0.6~2.1V, and the number of scan cycles is 8~10. According to the linear relationship between the concentration of CAF and its oxidation peak current, the concentration of CAF in each functional beverage is determined. , and the standard addition method was used to measure the standard addition recovery rate, and the measurement results are shown in Table 1. It can be concluded from the test results that the modified electrode can detect CAF in commercial cola and functional drinks, and the recovery rate is between 96...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com