Self-folding structure-containing polyurethane and preparation method thereof
A polyurethane and self-folding technology, which is applied in the field of polyurethane, can solve problems such as the decline in mechanical properties of polyurethane and insufficient hydrogen bonding in rigid segments, and achieve the effects of improved mechanical properties and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 10g of 1,5-naphthalene diisocyanate and 5.81g of ethanolamine into 100ml of N,N-dimethylformamide, continue to stir and react at room temperature for 0.5h, filter with suction and dry to obtain a hydroxyl-terminated naphthalene ring chain extender.
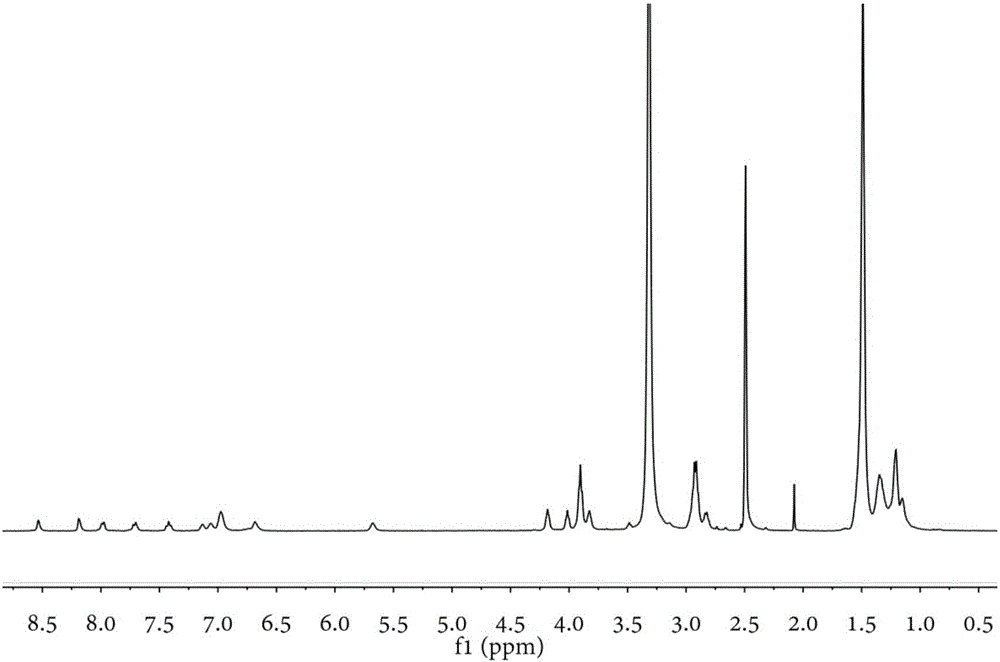
[0044] Characterized by H NMR spectrum, the NMR data are as follows:
[0045] δ / ppm=3.16-3.25(m,2H),3.45-3.58(m,2H),4.76-4.84(m,2H),6.71-6.79(m,2H),7.39-7.48(m,2H),7.71 -7.77(d,J=8.55Hz,2H),7.99-8.05(d,J=7.77Hz,2H),8.55(s,2H).
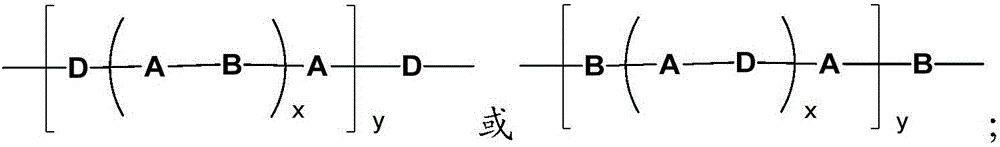
[0046] The product obtained is of formula I:
[0047]
[0048] Add 10 g of pyromellitic dianhydride and 5.0 g of ethanolamine into 100 ml of N,N-dimethylformamide, stir and react at room temperature for 12 hours to generate an amic acid solution, add 30 ml of toluene to the above amic acid solution, and raise the temperature to At 170°C, the water generated by the cyclization of amic acid is continuously separated through the water separator. When the separated water reaches the metered val...
Embodiment 2
[0060] Add 10g of 1,5-naphthalene diisocyanate and 7.14g of ethanolamine into 100ml of N,N-dimethylacetamide, continue to stir and react at room temperature for 0.5h, filter with suction, and dry to obtain a hydroxyl-terminated naphthalene ring chain extender. The product structure is as formula III:
[0061]
[0062] Add 10g of pyromellitic dianhydride and 6.9g of propanolamine into 100ml of N,N-dimethylacetamide, stir and react at room temperature for 12h to generate an amic acid solution, add 30ml of toluene to the above-mentioned amic acid solution, and lower the temperature Raise to 170°C, and continuously separate the water generated by the cyclization of amic acid through the water separator. After the separated water reaches the metered value, stop the reaction, and remove the toluene and N,N-dimethylacetamide in the system by distillation under reduced pressure , drying to obtain a hydroxyl-terminated imide ring chain extender, and the obtained product structure is...
Embodiment 3
[0069] Add 10g of 1,5-naphthalene diisocyanate and 5.81g of ethanolamine into 100ml of dimethyl sulfoxide, continue to stir and react at room temperature for 0.5h, filter with suction, and dry to obtain a hydroxyl-terminated naphthalene ring chain extender. The structure of the obtained product is as follows: V:
[0070]
[0071] Add 10g of pyromellitic dianhydride and 5.0g of ethanolamine into 100ml of N,N-dimethylacetamide, stir and react at room temperature for 12 hours to generate an amic acid solution, add 30ml of toluene to the above amic acid solution, and raise the temperature to 170 ℃, the water generated by the cyclization of amic acid is continuously separated through the water separator. When the separated water reaches the measured value, the reaction is stopped, the toluene and N,N-dimethylformamide in the system are removed by vacuum distillation, and dried Obtain the imide ring chain extender of hydroxyl termination, the product of gained is such as formula ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com