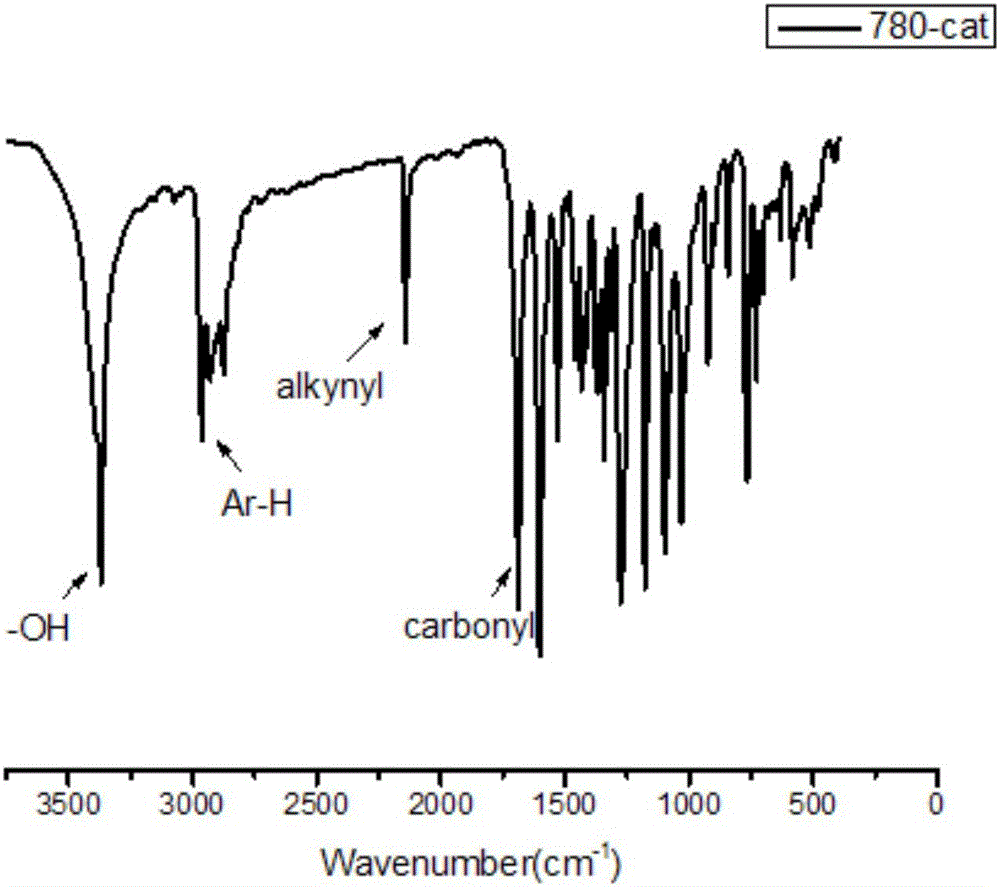
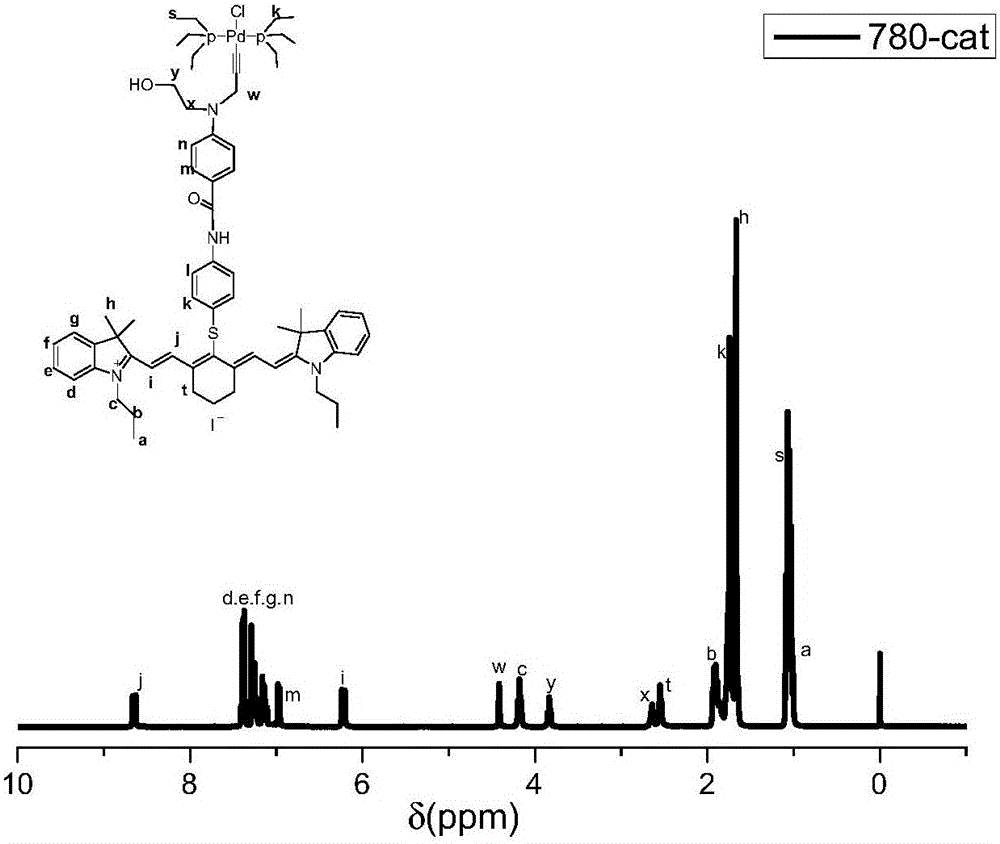
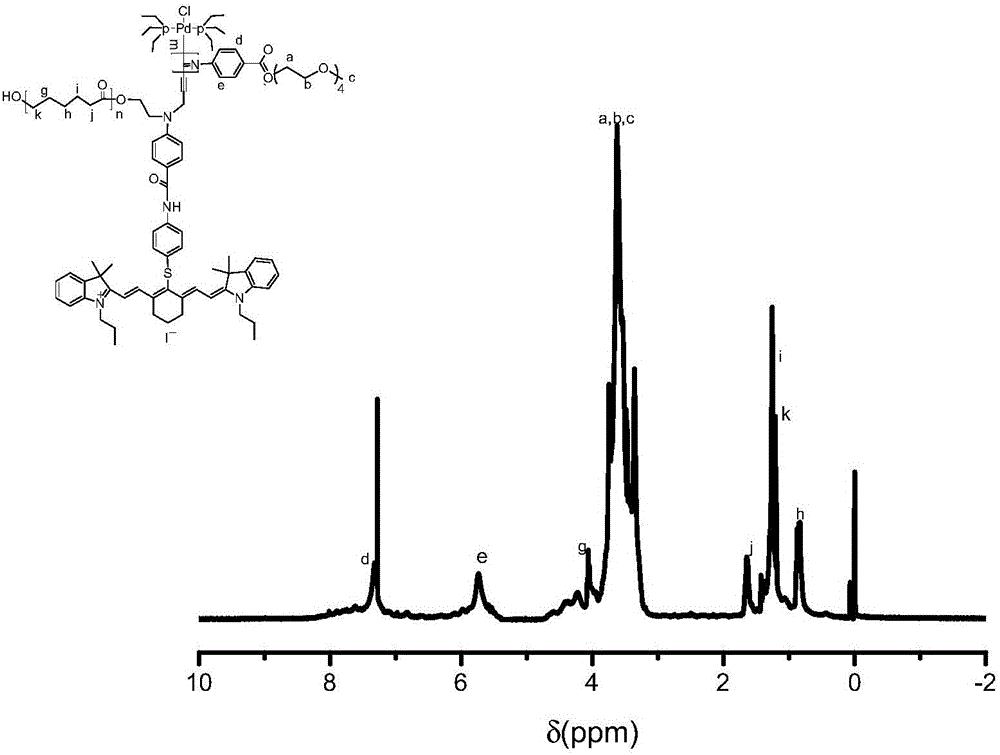
Synthesis and application of bifunctional initiator with near-infrared absorption and self-sorting polymerization characteristics
A self-classifying polymerization, bifunctional technology, applied in palladium organic compounds, platinum group organic compounds, organic chemistry, etc., can solve the problems of difficult implementation of hybrid polymerization, and achieve narrow molecular weight distribution, good stability, and monomer conversion rate. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of IR780 initiator
[0050] The iodide of IR780 and excess p-mercaptoaniline (added at a molar ratio of 1:1.02) were reacted in DMF solution at room temperature for 24 hours, and after being spin-dried, silica gel column chromatography (methanol: dichloromethane = 1:20), Intermediate I was obtained after drying
[0051]
[0052] Add p-aminobenzoic acid, anhydrous potassium carbonate, and propyne bromide in a molar ratio of 1:(1-3):1.2 to dry acetonitrile, stir and react at room temperature for 24 hours in a nitrogen atmosphere, wash with water, and saturate NaHCO 3 washing, salt washing, separation of the organic phase, spin-drying, the crude product is purified by silica gel column chromatography (eluent by volume: petroleum ether: ethyl acetate=4:1), and after drying, intermediate II is obtained. The structural formula is as follows:
[0053]
[0054] Add the intermediate IV, potassium carbonate, and potassium iodide to dry acetonit...
Embodiment 2
[0065] Embodiment 2: Initiate the polymerization reaction of hydrophilic benzene isocyanide
[0066] The polymerization of benzyl isonitrile is carried out under anhydrous and oxygen-free conditions. Add 7.63 μmol (8.21 mg) of the initiator prepared in Example 1 and 0.55 mmol (160.0 mg) of benzyl isonitrile monomer to a 10 mL polymerization bottle, and repeat by vacuumizing and filling with nitrogen 3 times, add 2.0 mL of dry chloroform, reflux at 60°C for 20 hours, add 10 mL of n-hexane to quench, precipitate the polymer, wash 5 times with n-hexane, centrifuge to obtain a yellow flocculent precipitate, vacuum dry to mass constant. Obtain 150.1mg polyisocyanide, its number-average molecular weight is 2.12×10 4 , the molecular weight distribution index is 1.09.
[0067] The structural formula of the benzene isonitrile monomer in the present embodiment is:
[0068]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com