Application of piperidinium-2,3-dihydroxy-5-((2R,3R)-3,5,7-trihydroxy-4-oxochroman-2-yl)phenolate (PDTOP) in preparation of medicines for treating or preventing diseases of respiratory system
A technology for respiratory diseases and phenol theophyllic acid, applied in the field of phenolic theophyllic acid and its preparation and application, can solve problems such as unknown mechanism of action, and achieve the effect of preventing the spread of influenza and preventing invasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1. preparation of phenol theophyllic acid
[0032] The phenol theophyllic acid (PDTOP) of the present invention is a synthetic flavonoid compound. Also known as phenol dihydromyricetin (English name: Piperidinium2,3-dihydroxy-5-((2R,3R)-3,5,7-trihydroxy-4-oxochroman-2-yl)phenolate, PDTOP)
[0033] The chemical formula is:
[0034] The preparation method of phenol theophyllic acid of the present invention:
[0035] Add five millimoles (5mmol, 1.6g) of dihydromyricetin to 20 milliliters of methanol and dissolve for 30 minutes, place the dissolved solution in an ice bath, and titrate with 5.5 millimoles of piperidine in the presence of argon at -10°C Add dihydromyricetin methanol solution, leave it for 20 minutes to make it react completely, then, concentrate the reaction solution under low pressure vacuum situation, after filtering, wash three times with methanol to obtain phenotheophylline (about 2g).
[0036] The preparation method of dihydromyricetin: ...
Embodiment 2
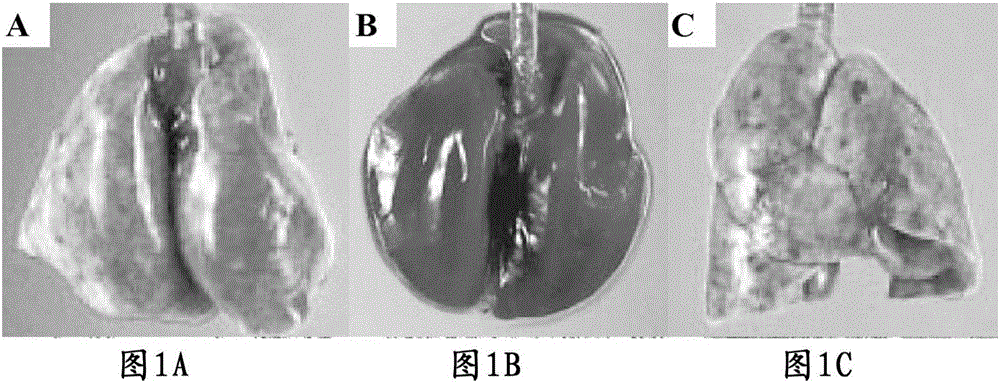
[0043] Embodiment 2. The animal experiment of preventing and treating smog
[0044] Experimental animals: conventional experimental white mice, male / female, with a body weight of 300 / 275 grams, respectively. 6 groups;
[0045] Experimental drug: phenol theophyllic acid;
[0046] Experimental drug dosage and configuration:
[0047] 1) 0.5 mg / kg body weight; or
[0048] or
[0049]3) Put 1 gram of agar into 100 milliliters of experimental water, boil it, add 0.05 grams of phenol theophyllic acid, stir well, let it cool until it becomes frozen. Give 1cm 3 Agar jelly (containing 0.5 mg phenol theophyllic acid element). or
[0050] 4) Measuring metabolic rate: 1cm 3 Agar jelly contains 0.2 grams of phenol theophyllic acid. or
[0051] 5) For toxicity determination: 1cm 3 Agar jelly contains 0.2 grams of phenol theophyllic acid.
[0052] (Note: at 500mg / 1cm 3 Give 75000 grams of body weight as the standard, 500 mg / 75000 grams of body weight=x / 300 grams of experimental w...
Embodiment 3
[0064] Embodiment 3. The experiment of phenol theophyllic acid anti-influenza A (H1N1) virus infection
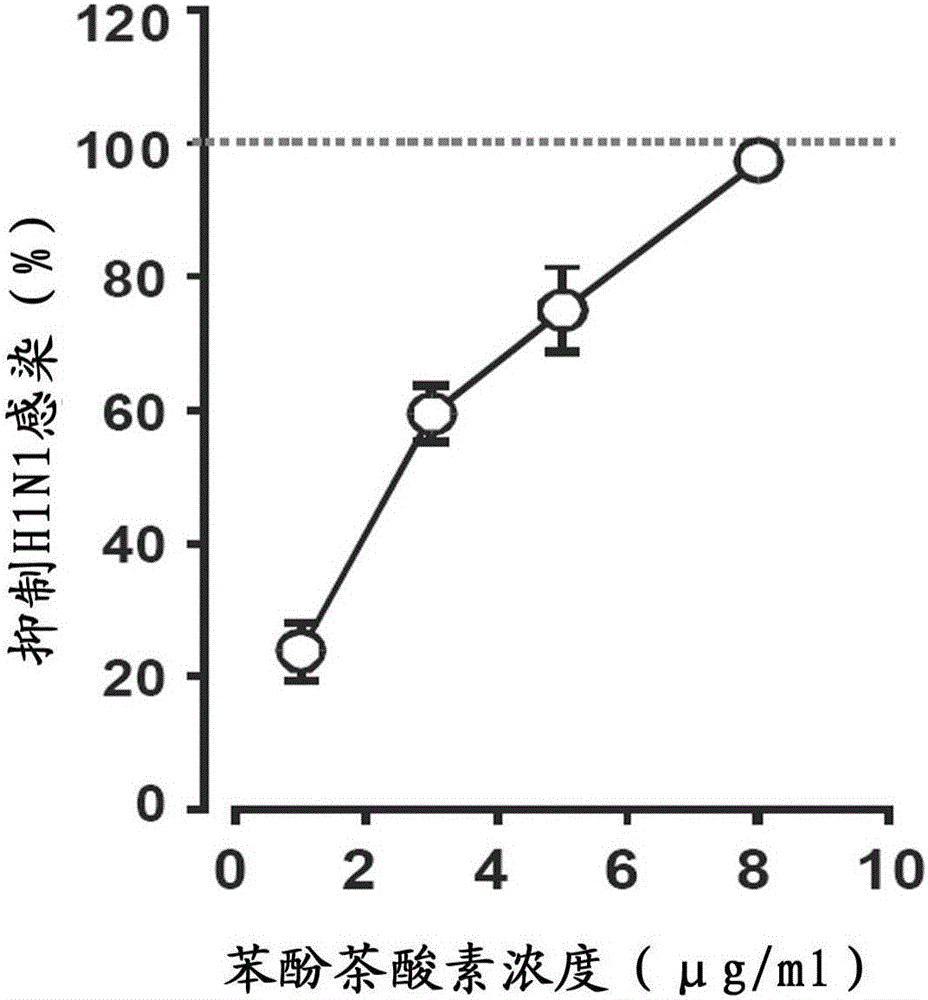
[0065] Influenza A (H1N1) virus 252 (± 34) micrograms per milliliter of particles was used to determine the antiviral effect of phenol theophylline. H1N1 virus particles were co-incubated with dog kidney cells for a total of 2 hours in a medium containing phenocin and washed 3 times. Filter through a filter and remove unbound compounds with common culture medium. Virus particles were harvested and analyzed using a direct binding assay—the phenolic acid-bound and unbound fractions of the virus were then compared.
[0066] The results showed that phenol theophyllic acid had obvious dose-dependent inhibitory effect on H1N1 virus infection ( figure 2 ). 50% inhibitory concentration (IC) of phenol theophyllic acid to H1N1 50 ) is 2.4 micrograms / ml (8μM), and 100% inhibits the concentration of H1N1 virus infection (IC 100 ) micrograms / ml (27 μM).
[0067] The mechanism of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com