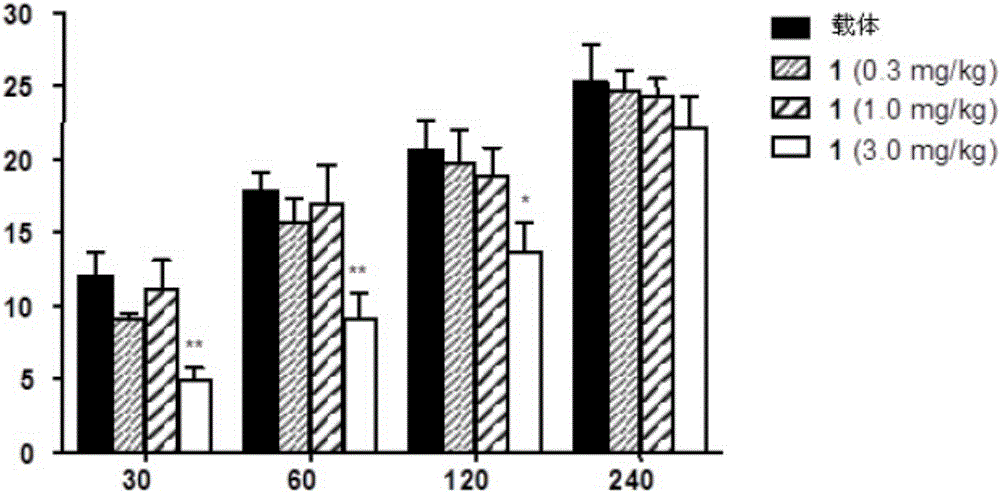
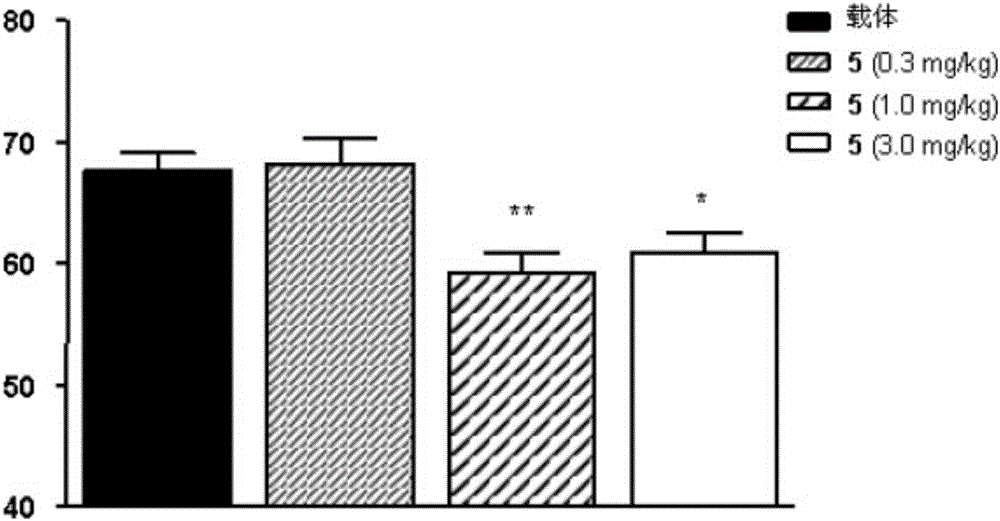
Oxazolidinone derivatives as PPAR ligands
A technology of oxazolidine and alkyl, applied in the field of oxazolidinone derivatives used as PPAR ligands, can solve problems such as liver damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Embodiment 1. Preparation of (R)-3-((4-benzyl-2-oxooxazolidin-3-yl)methyl)-N-(4-((4-( tert-butyl)benzyl)carbamoyl)phenyl)benzamide (1)
[0158]
[0159] in N 2 Under the atmosphere, take the Al(CH 3 ) 3 2.0 M heptane (0.18 mL, 0.36 mmol) was added to (R)-methyl 3-((4-benzyl-2-oxooxazolidin-3-yl)methyl)benzoate (0.06 g , 0.18 mmol) and compound 4-amino-N-(4-(tert-butyl)benzyl)benzamide (0.10 g, 0.36 mmol); the reaction mixture was heated at 125°C in a microwave reactor 35 minutes. The crude reaction product was cooled in an ice-water mixture and acidified with HCl (1N) until effervescence ceased. Then, it was extracted with diethyl ether (20 mL), and the organic extract was washed with anhydrous MgSO 4 Dry, and remove the solvent under reduced pressure. The crude reaction product was purified by biotage chromatography (Hex / AcOEt) to afford compound 1 as a white solid (0.05 g, 44%). 1 H NMR (300MHz, CDCl 3 )δ8.91(s,1H,NH),7.83(m,1H),7.78–7.63(m,5H),7.44–7.15(m...
Embodiment 2
[0168]Example 2. Preparation of (R)-3-((4-benzyl-2-oxooxazolidin-3-yl)methyl)-N-(4-((4-( Trifluoromethyl)benzyl)carbamoyl)phenyl)benzamide (2)
[0169]
[0170] in N 2 Under the atmosphere, take the Al(CH 3 ) 3 2.0 M heptane (0.30 mL, 0.61 mmol) was added to (R)-methyl 3-((4-benzyl-2-oxooxazolidin-3-yl)methyl)benzoate (0.10 g , 0.31 mmol) and 4-amino-N-(4-(trifluoromethyl)benzyl)benzamide (0.18 g, 0.61 mmol); the reaction mixture was heated at 125°C in a microwave reactor 35 minutes. The crude reaction product was cooled in an ice-water mixture and acidified with HCl (1N) until effervescence ceased. Then, it was extracted with diethyl ether (30ml), and the organic extract was washed with anhydrous MgSO 4 Dry, and remove the solvent under reduced pressure. The crude reaction product was purified by chromatography (6:4 hexane / ethyl acetate) to afford compound 2 as a white solid (0.05 g, 44%). 1 H NMR (300MHz, DMSO-d 6 )δ10.49(s,1H,NH),9.07(t,J=6.1Hz,1H,CH 2 NH ),7...
Embodiment 3
[0175] Example 3. Preparation of (R)-3-((4-benzyl-2-oxooxazolidin-3-yl)methyl)-N-(4-(benzylaminomethyl) of formula (VI) Acyl)phenyl)benzamide (3)
[0176]
[0177] in N 2 Under the atmosphere, take the Al(CH 3 ) 3 2.0 M heptane (0.18 mL, 0.36 mmol) was added to (R)-methyl 3-((4-benzyl-2-oxooxazolidin-3-yl)methyl)benzoate (0.06 g , 0.18 mmol) and compound 4-amino-N-benzylbenzamide (0.081 g, 0.36 mmol) in anhydrous THF solution (10 mL); the reaction mixture was heated in a microwave reactor at 125 °C for 35 min . The crude reaction product was cooled in a mixture of ice and water, and HCl (1 N) was added dropwise until effervescence ceased. Then, it was extracted with diethyl ether (20 mL), and the organic extract was washed with anhydrous MgSO 4 Dry, and remove the solvent under reduced pressure. The crude reaction product was purified by chromatography (6:4 hexane / ethyl acetate) to afford 3 as a white solid (0.04 g, 40%). 1 H NMR (300MHz, DMSO-d 6 )δ10.47(s,1H,NH),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com