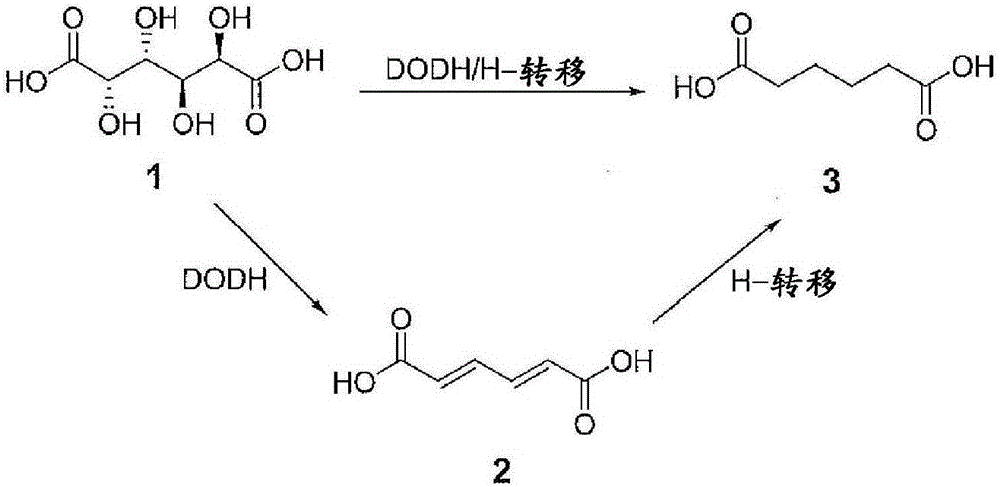
Chemical process to convert mucic acid to adipic acid
A technology of adipic acid and muconic acid, applied in the field of synthesizing saturated polycarboxylic acid esters, can solve the problems of low HMF conversion rate and insufficient reaction selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Materials
[0075]All starting materials were commercially available and used as received unless otherwise indicated. Muconic acid (98%), 3-pentanol (98%), TsOH (98%), 2-propanol (99.9%) were purchased from Merck; trans, trans-muconic acid (98%), 3- Octanol (99%) and 5% Pt / C were purchased from Aldrich. Methyltrioxyrhenium (MTO) (98%), Re 2 o 7 (99.99%) and Re 2 (CO) 10 was purchased from Strem Chemical, USA; 1-butanol (99.5%) was purchased from BDH Laboratory Supplies, England. Other reagents involved were from Sigma or Merck. Obtained using Brucker AV-400 (400MHz) spectrometer 1 H and 13 C NMR spectra. Chemical shifts are reported in ppm with reference to tetramethylsilane with solvent resonance as internal standard.
Embodiment 2
[0076] Example 2: General procedure for the synthesis of diethyl mucate 6
[0077] Mucic acid (5.0 g), H 2 SO 4 A mixture of (1ml) and ethanol (150.0ml) was refluxed (80°C) for 24h. The reaction mixture was cooled to room temperature and then stored at 3°C for 1 day. The white precipitate was filtered off, washed with a small amount of cold ethanol, and dried under vacuum at 50 °C overnight. The mother liquor was evaporated to dryness to give a brown solid. The solid obtained from the mother liquor was recrystallized in 10 ml ethanol and recovered by the procedure as described above. The total amount of diethyl mucate was 5.1 g (90.0% yield).
Embodiment 3
[0078] Example 3: General Procedure for Deoxygenation Dehydration (DODH) of Mucic Acid
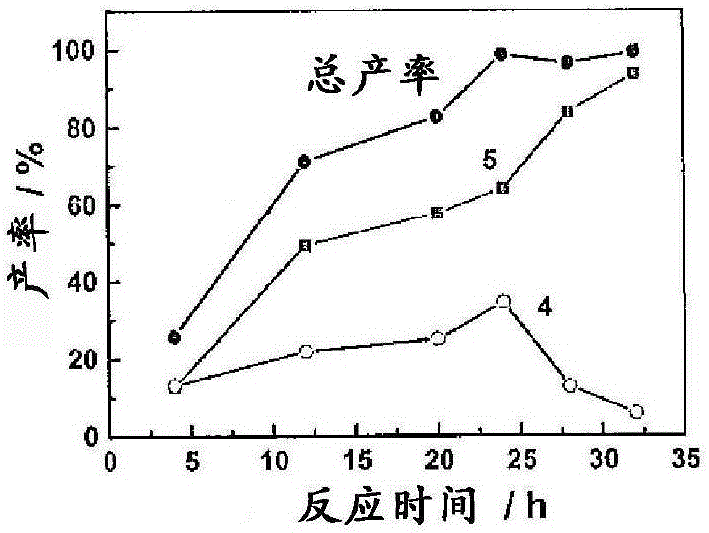
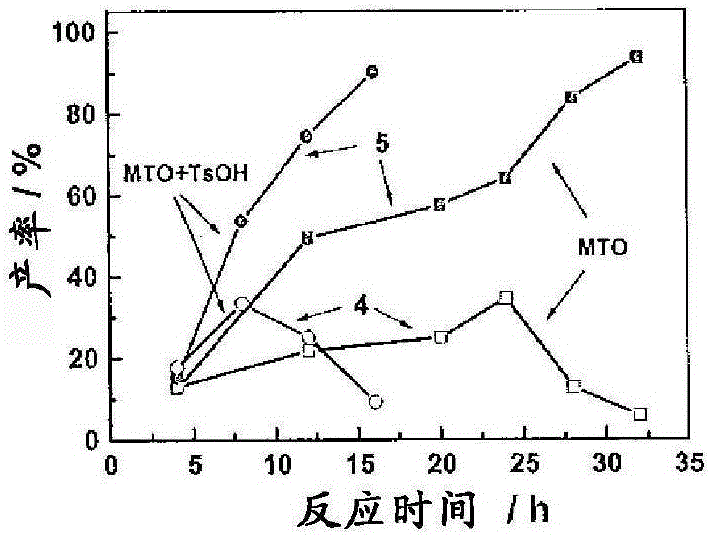
[0079] A mixture of mucic acid (1mmol, 210mg), methyl trioxyrhenium (MTO) (0.05mmol, 12mg), TsOH (0.05mmol, 12mg) and 3-pentanol (20.0ml) was placed in a flowing 50ml flask air or N 2 Under reflux (120°C). The mixture was initially a white suspension, then turned into a brown and clear solution after 4 h. After 12 h, the reaction mixture was evaporated to dryness. The solid was recrystallized to give the product. For kinetic studies, 1 ml of the reaction mixture was taken at specific time intervals and dried for NMR analysis; a known amount of mesitylene was added as an internal standard.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com