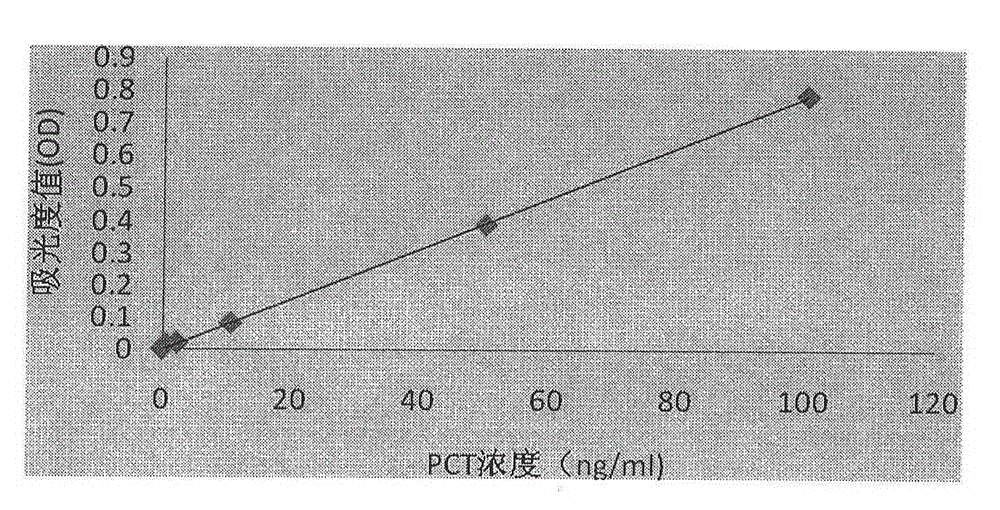
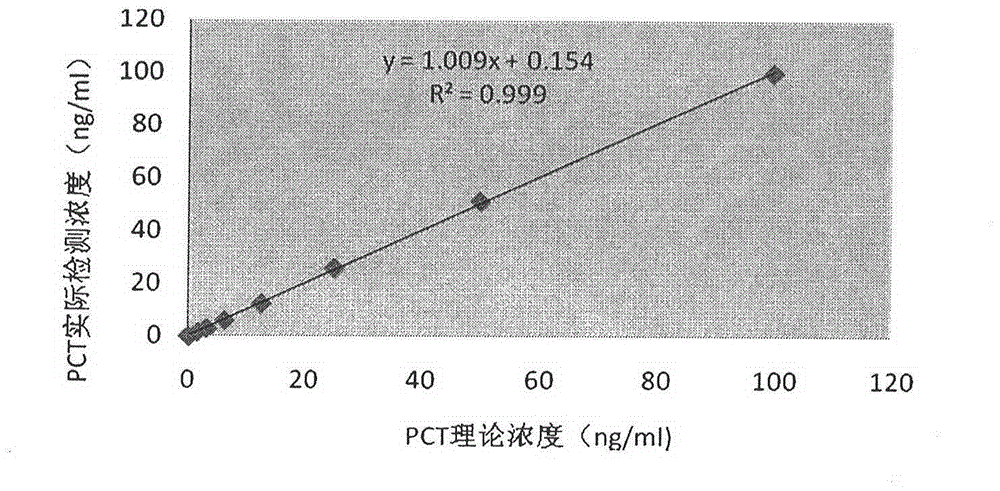
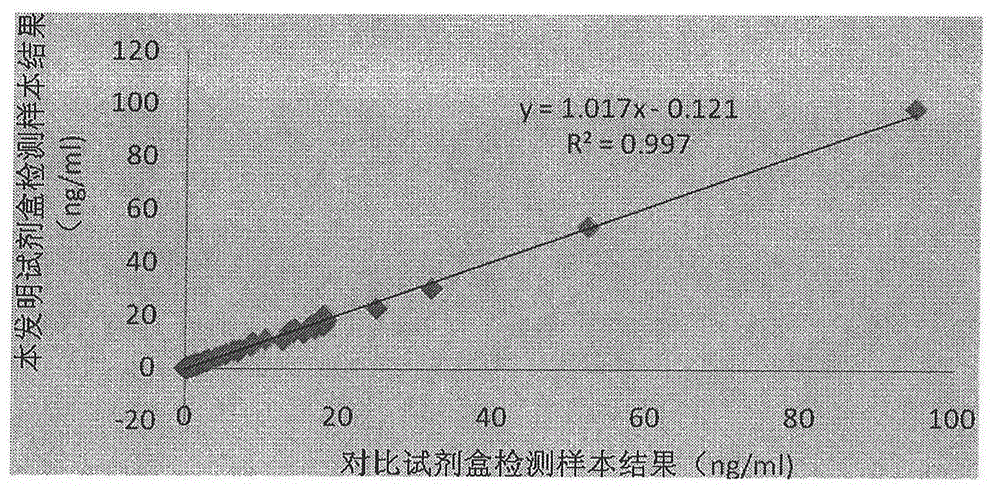
Procalcitonin collaurum immune colorimetric determination detection kit and preparation method thereof
A technology for detecting kits and procalcitonin, which is applied in the direction of biological testing, material inspection products, etc., can solve the problems of complicated operation, expensive, time-consuming, etc., and achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of the reagent R2 in this example is divided into three steps. First, a solution of gold nanoparticles (i.e., colloidal gold) is prepared, and then the antibody is coupled to the gold nanoparticles, and finally mixed with a buffer, a stabilizer, a coagulant, and a preservative. Miscible into a complete system.
[0041] The specific process is as follows:
[0042] (1) Commercially available chloroauric acid and trisodium citrate were respectively prepared into 1% aqueous solution, boiled 1L of deionized water or ultrapure water, then added 10mL of chloroauric acid solution and kept boiling for about 1min, then added 10mL The trisodium citrate solution, as time changes, the solution boils again, and the color of the solution changes from bright yellow to purple black, and then turns to wine red for a short time, and then keeps boiling for 10 minutes, cools, replenishes water to the original volume, and sets aside .
[0043] The prepared colloidal gold so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com