Albumin nanoparticles realizing co-delivery of antitumor drug and MRI (magnetic resonance imaging) contrast medium and preparation method of albumin nanoparticles
A technology of albumin nanoparticles and anti-tumor drugs, applied in anti-tumor drugs, MRI/magnetic resonance imaging contrast agents, pharmaceutical formulations, etc. To avoid toxicity and reverse tumor drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] (1) BSA-MnO 2 Solution preparation: under stirring, Na at a constant rate 2 S 2 o 3 The solution was dropped into KMnO 4 In the solution, react under nitrogen protection at 4-30 °C for 1-4 hours to obtain MnO 2 Colloidal nanoparticle solution; MnO 2 Drop BSA solution into the colloidal nanoparticle solution, react for 6-24 hours at 4-30°C under nitrogen protection, and purify by dialysis with pure water to obtain a stable brown colloidal solution, namely BSA-MnO 2 solution;
[0043] (2) Desolvation-chemical cross-linking method to entrap antineoplastic drugs: add the antineoplastic drug solution to BSA-MnO dropwise according to a certain ratio 2 Add a certain volume of absolute ethanol dropwise to the solution, stir at 4-30°C for 10-60 minutes, add a cross-linking agent, seal and stir at 4-30°C for 6-24 hours, distill under reduced pressure to remove ethanol, and dialyze and purify That is, the antineoplastic drug and the MRI contrast agent co-deliver the albumin...
Embodiment 1
[0044] Embodiment 1: the preparation of BMDN
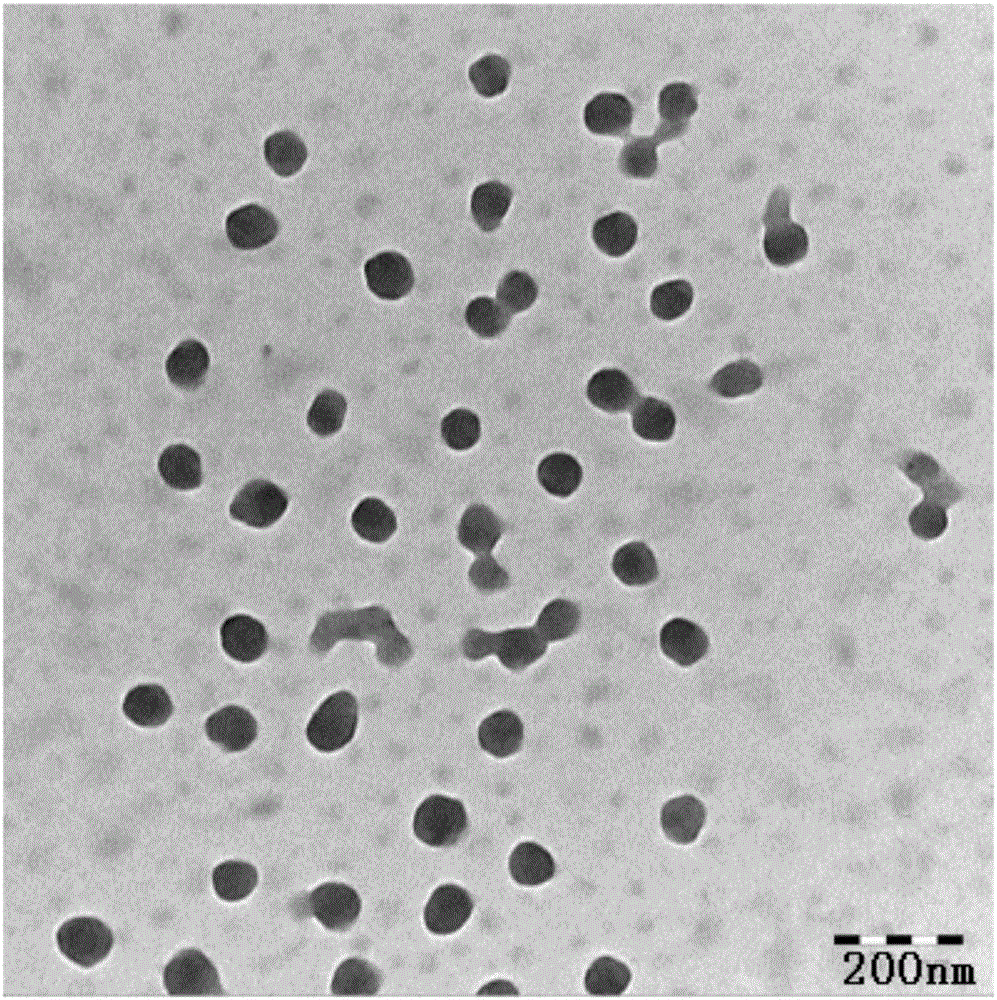
[0045] Prepare 0.3 mg / mL of Na 2 S 2 o 3 solution and 0.8 mg / mL of KMnO 4 Solution, 20mL Na 2 S 2 o 3 The solution was dropped into 10 mL KMnO 4 The solution was stirred at room temperature for 1 hour under the protection of nitrogen. 7.5 mL of BSA (25 mg / mL) was added dropwise to the reaction solution, and stirred at room temperature for 12 hours under nitrogen protection. The resulting product was dialyzed against pure water 4 times for half an hour each time.
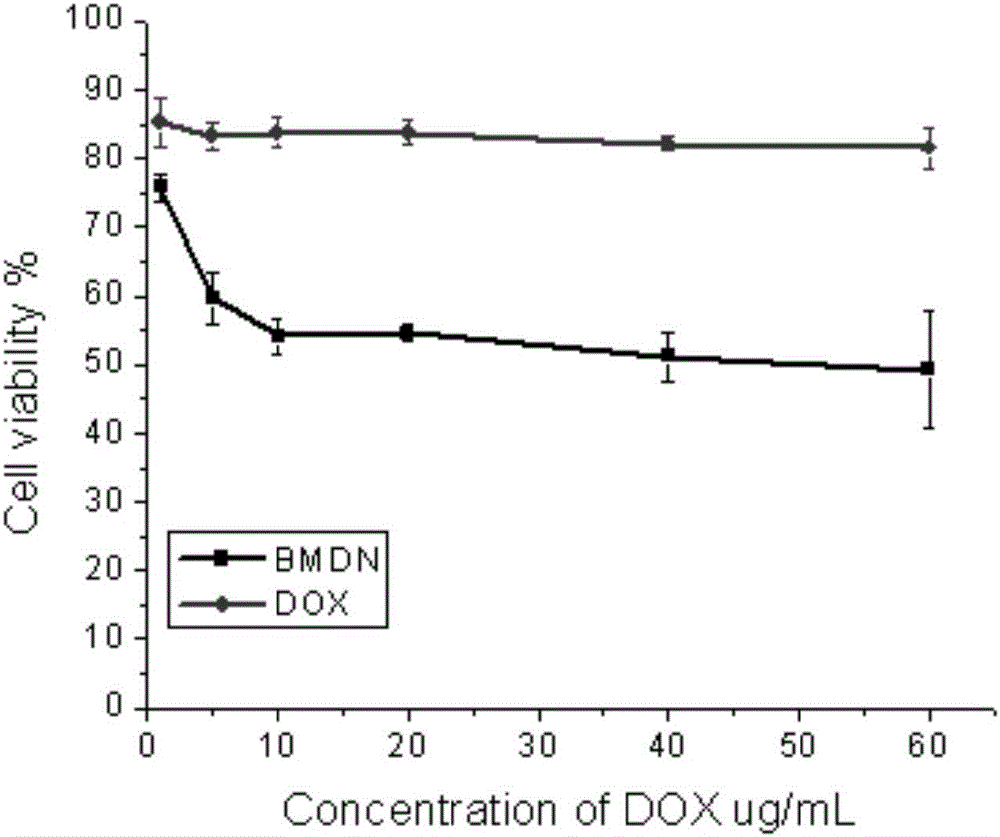
[0046] Under stirring, DOX solution (5 mg / mL) was added dropwise to BSA-MnO according to the theoretical drug loading of 10%. 2 solution, and then immediately add 1 volume of absolute ethanol dropwise to the above solution. After stirring for 15 minutes, 0.25% glutaraldehyde was added to the system (0.367 μL glutaraldehyde per mg of BSA), the reaction bottle was sealed, and stirring was continued for 12 hours at room temperature. Ethanol was distilled off unde...
Embodiment 2
[0047] Example 2: BMDN pH-responsive drug release
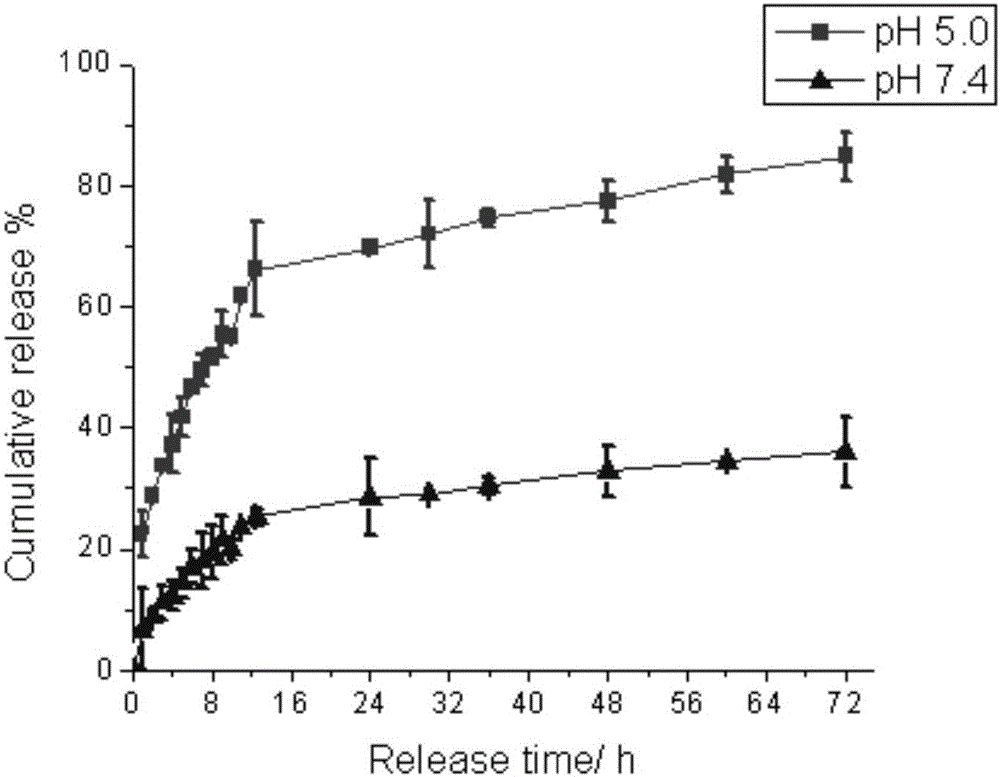
[0048] Take 1.5 mL of BMDN solution in a dialysis bag, tie the bag tightly, and submerge it in an Erlenmeyer flask filled with 28.5 mL of phosphate buffer solution (pH 5.0 or 7.4). Then the Erlenmeyer flask was placed in a constant temperature shaker at 37 °C, shaking at 120 rpm. At specific time points, 3 mL of release medium was taken to measure the absorbance at 479 nm, and 3 mL of fresh phosphate buffer solution was added. Calculate the concentration of DOX in the release medium according to the standard curve, thereby calculating the release degree and drawing the release curve, the results are as follows figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com