A cyclosuccinamide compound with antitumor activity and its preparation method and application
A technology of cyclosuccinamide and anti-tumor activity, applied in the field of biomedicine, can solve problems such as hair loss and chemical drugs that cannot achieve therapeutic effects, and achieve the effect of inhibiting proliferation and migration, inhibiting growth and migration, and inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
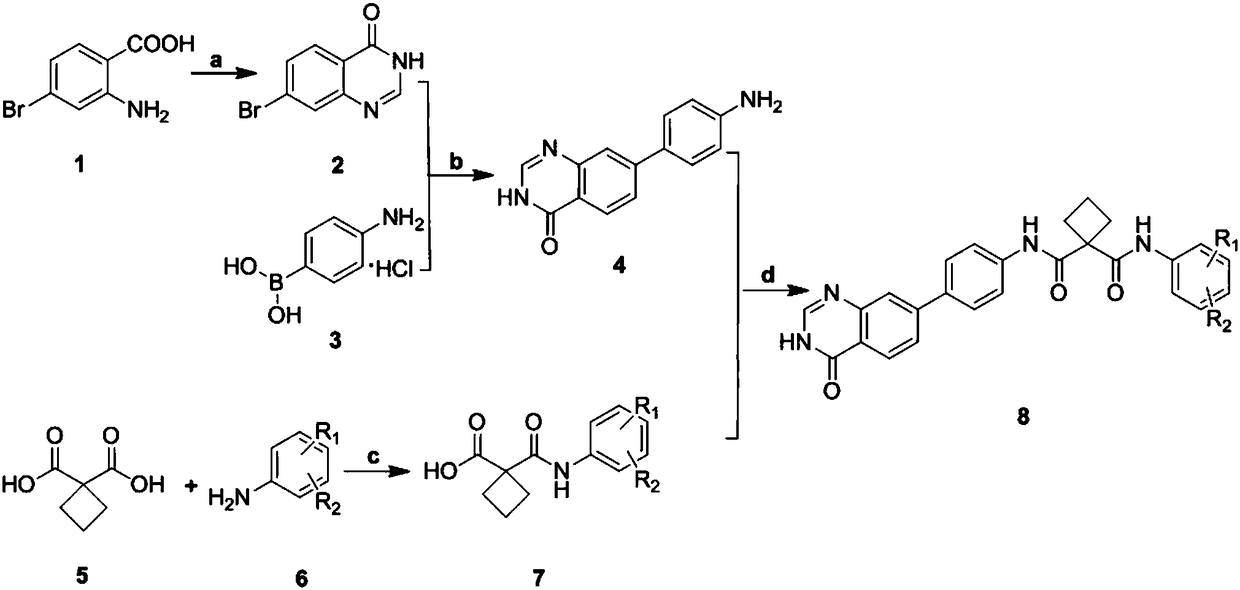
[0035] In the structural formula of the cyclosuccinamide compound with antitumor activity, R 1 for hydrogen, R 2 For F, prepared by the following steps (see figure 1 ):
[0036] 1) Preparation of 7-bromoquinazolin-4(3H)-one (compound 2) by cyclization reaction of 2-amino-4-bromobenzoic acid (compound 1) and formamide
[0037] Dissolve 5.0g (23.14mmol) of 2-amino-4-bromobenzoic acid in 80ml (2.01mmol) of formamide, and react for 1.45h under nitrogen protection at 150°C and a microwave power of 300W. After the reaction, the The reaction solution was added to ice water, extracted 3-4 times with ethyl acetate, and the extracted organic phase was washed with saturated sodium bicarbonate solution and saturated brine successively, then dried with anhydrous sodium sulfate, and then spin-dried to obtain a reddish-brown residue , the reddish-brown residue was separated by chromatography to obtain 1.89 g of reddish-brown solid 7-bromoquinazolin-4(3H)-one with a yield of 36.3%.
[003...
Embodiment 2
[0050] In the structural formula of the cyclosuccinamide compound with antitumor activity, R 1 , R 2 Both are trifluoromethyl.
[0051] Step 1) to step 3) are the same as step 1) to step 3) in Example 1, that is to prepare 7- Bromoquinazolin-4(3H)-one (compound 2), 7-bromoquinazolin-4(3H)-one (compound 2) and p-aminophenyl borate hydrochloride (compound 3) were obtained by Suzuki reaction 4((3H)-7-quinazolin-4-one)aniline (compound 4), 1,1 cyclobutyldicarboxylic acid (compound 5) and 3,5-ditrifluoromethylaniline (compound 6) in The acylation reaction under the condition of thionyl chloride gave 1-((3,5-bistrifluoromethylphenyl)carbamoyl)cyclobutanecarboxylic acid (compound 7).
[0052] 4) 4((3H)-7-quinazolin-4-one)aniline (compound 4) and 1-((3,5-bistrifluoromethylphenyl)carbamoyl)cyclobutyric acid (compound 7 ) generates the target compound (compound 8) under the condensation of HATU condensing agent, and the specific operation steps are:
[0053] Under ice-bath conditio...
Embodiment 3
[0060] In the structural formula of the cyclosuccinamide compound with antitumor activity, R 1 is chlorine, R 2 for CH 3 .
[0061] Step 1) to step 3) are the same as step 1) to step 3) in Example 1, that is to prepare 7- Bromoquinazolin-4(3H)-one (compound 2), 7-bromoquinazolin-4(3H)-one (compound 2) and p-aminophenyl borate hydrochloride (compound 3) were obtained by Suzuki reaction 4((3H)-7-quinazolin-4-one)aniline (compound 4), 1,1 cyclobutyldicarboxylic acid (compound 5) and 3-chloro-4-methylaniline (compound 6) in chlorine Acylation under sulfoxide conditions gave 1-((3-chloro-4-methylphenyl)carbamoyl)cyclobutanecarboxylic acid (compound 7).
[0062] 4) 4((3H)-7-quinazolin-4-one)aniline (compound 4) and 1-((3-chloro-4-methylphenyl)carbamoyl)cyclobutyric acid (compound 7) Generate the target compound (compound 8) under the condensation of HATU condensing agent, and the specific operation steps are:
[0063] Under ice-bath condition, 0.59g (2.49mmol) 4 ((3H)-7-quinaz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com