Basic protein entrapment vector, preparation method of entrapment vector and entrapment method
A protein and alkaline technology, which is applied in biochemical equipment and methods, peptide/protein components, pharmaceutical formulations, etc., can solve the problems of high-efficiency entrapment of basic proteins, difficulty in achieving a large amount of entrapment, etc., and achieve easy entrapment or modification, low equipment cost, good research and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] This embodiment relates to a method for encapsulating chymotrypsin in calcium carbonate microspheres doped with sodium heparin.
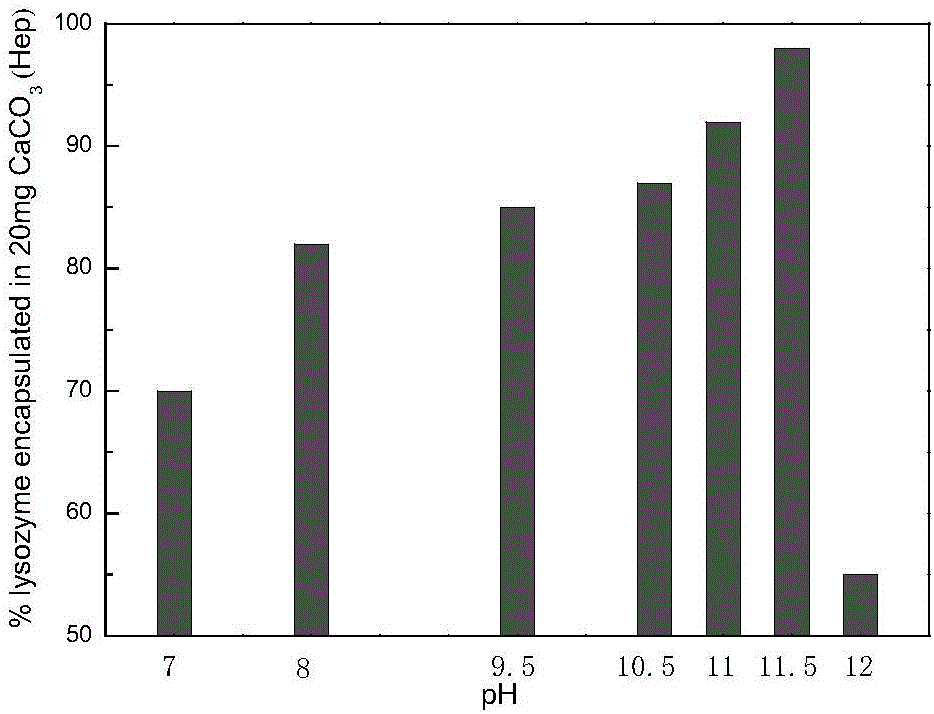
[0033] (1) Mix 2ml of calcium chloride solution with a concentration of 0.5M and 1ml of sodium heparin with a concentration of 6mg / ml, stir for 1~5min, then quickly add 3ml of carbonic acid with a concentration of 0.33M at 900 rpm Sodium solution, stirred for 35-40s, let stand for 15min, centrifuged to collect the calcium carbonate precipitate containing heparin sodium, washed 3 times with water. The prepared calcium carbonate particles containing heparin sodium have a diameter of about 2-5 μm.
[0034] (2) Take an appropriate amount of the above-mentioned calcium carbonate particles containing heparin sodium and place them in a 1.5ml centrifuge tube, add 1ml of chymotrypsin solution (pH=7.2) with a concentration of 1mg / ml, incubate at 4°C for 30min, and centrifuge , lyophilized to obtain heparin sodium calcium carbonate particles loaded wit...
Embodiment 2
[0036] This embodiment relates to a method for encapsulating lysozyme in calcium carbonate microspheres doped with sodium heparin.
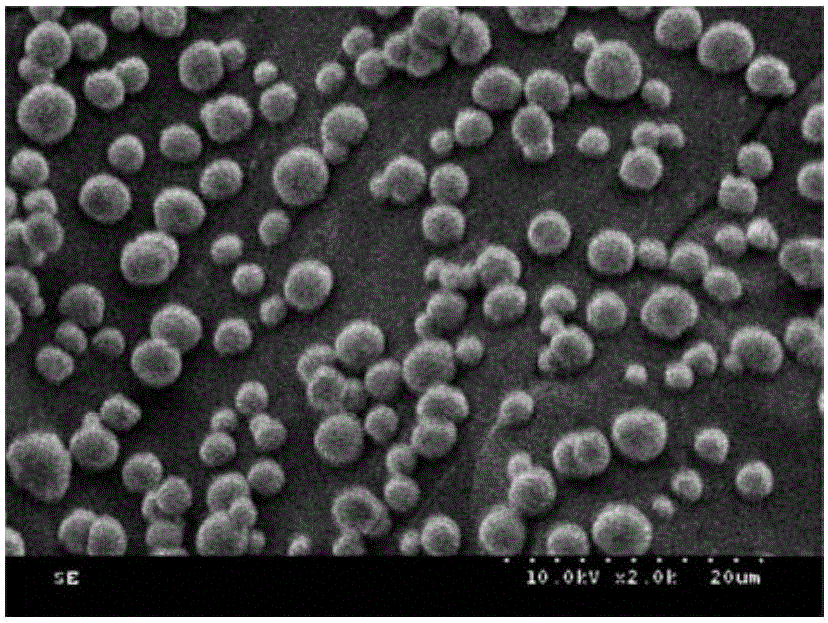
[0037](1) After mixing 2ml of calcium chloride solution with a concentration of 0.5M and 1ml of heparin sodium with a concentration of 6mg / ml, stir for 1~5min, then quickly add 3ml of sodium carbonate with a concentration of 0.33M at 1000 rpm solution, stirred for 35-40s, and stood still for 25 minutes, and the generated calcium carbonate precipitate containing heparin sodium was collected by centrifugation, and washed with water for 3 times. The prepared calcium carbonate microspheres doped with sodium heparin have a diameter of about 2-5 μm, see figure 1 .
[0038] (2) Take an appropriate amount of calcium carbonate microspheres doped with heparin sodium and put them in a 1.5ml centrifuge tube, add 1ml of 1mg / ml lysozyme solution (pH=7.2), incubate at 10°C for 60min, and centrifuge , lyophilized to obtain lysozyme-doped calcium carbonate micr...
Embodiment 3
[0040] This embodiment relates to a method for entrapping lysozyme in calcium carbonate microspheres doped with dextran sulfate.
[0041] (1) Mix 2ml of calcium chloride solution with a concentration of 0.5M and 1ml of dextran sulfate with a concentration of 6mg / ml, stir for 1~5min, and then quickly add 3ml with a concentration of 0.33M at 1200 rpm Sodium carbonate solution, stirred for 35~40s, let stand for 20min, centrifuged to collect the calcium carbonate precipitate doped with dextran sulfate, washed 3 times with water. The prepared calcium carbonate particles doped with dextran sulfate have a diameter of about 2-5 μm.
[0042] (2) Take an appropriate amount of calcium carbonate doped with dextran sulfate and place it in a 1.5ml centrifuge tube, add 1ml of lysozyme solution (pH=7.2) with a concentration of 1mg / ml, and incubate at 25°C for 90min. After centrifugation and freeze-drying, dextran sulfate-doped calcium carbonate microspheres loaded with lysozyme can be obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com