Preparation method for preparing inactivated vaccine by means of Muscovy duck hepatitis virus
A Muscovy duck hepatitis and inactivated vaccine technology, which is applied in the direction of antiviral agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of stunted development of Muscovy ducks, affecting the economic benefits of Muscovy ducks, and low cure rate, etc. problems, to achieve the effects of stimulating the development of the body's immune cells, enhancing the body's immune function, and quickly absorbing the vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A preparation method of muscovy duck hepatitis virus recombinant chicken colony-stimulating factor adjuvant inactivated vaccine, which uses muscovy duck hepatitis virus GS14 strain as an immunogen and recombinant chicken colony-stimulating factor as an adjuvant, and its specific steps are as follows:
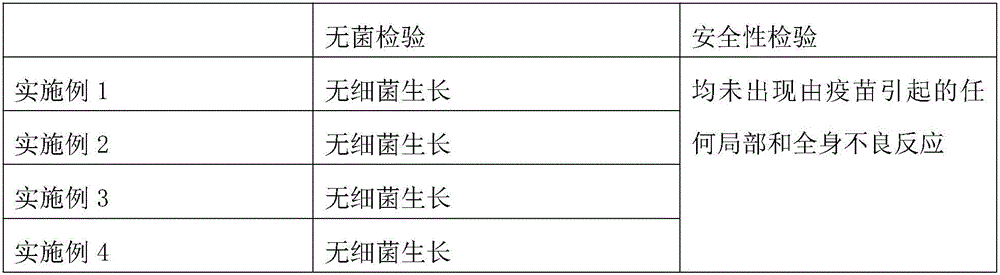
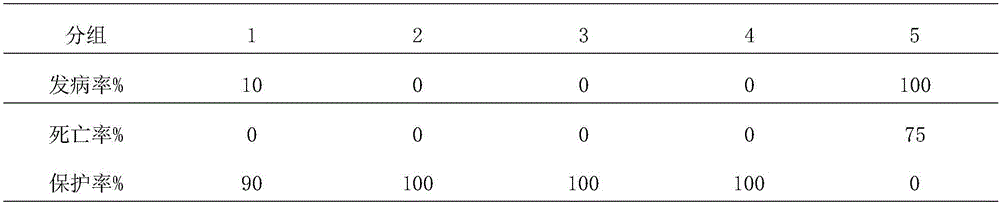
[0024] (1) Antigen preparation: Dilute Muscovy duck hepatitis virus GS14 strain 10,000 times with normal saline, inoculate 11-12-day-old susceptible Muscovy duck embryos through the allantoic cavity, 0.2ml / embryo, collect and inoculate and die within 60-168 hours Muscovy duck embryo allantoic fluid, if the sterility test is qualified, is the antigen, and then stored at -40°C;
[0025] (2) Antigen inactivation: the antigen in step (1) is added into a formaldehyde solution with a final volume concentration of 0.1%, and inactivated at 37°C for 24 hours, during which the antigen is shaken 4 times for 3 minutes each time; the formaldehyde solution The content of formaldehyde i...
Embodiment 2
[0028] A preparation method of muscovy duck hepatitis virus recombinant chicken colony-stimulating factor adjuvant inactivated vaccine, which uses muscovy duck hepatitis virus GS14 strain as an immunogen and recombinant chicken colony-stimulating factor as an adjuvant, and its specific steps are as follows:
[0029] (1) Antigen preparation: Dilute Muscovy duck hepatitis virus GS14 strain 5000 times with normal saline, inoculate 11-12 day-old susceptible Muscovy duck embryos through the allantoic cavity, 0.1ml / embryo, collect and inoculate and die within 60-168 hours Muscovy duck embryo allantoic fluid, if the sterility test is qualified, is the antigen, and then stored at -20°C;
[0030] (2) Antigen inactivation: the antigen in step (1) is added into a formaldehyde solution with a final volume concentration of 0.1%, and inactivated at 37°C for 24 hours, during which the antigen is shaken 4 times for 3 minutes each time; the formaldehyde solution The content of formaldehyde in ...
Embodiment 3
[0033] A preparation method of muscovy duck hepatitis virus recombinant chicken colony-stimulating factor adjuvant inactivated vaccine, which uses muscovy duck hepatitis virus GS14 strain as an immunogen and recombinant chicken colony-stimulating factor as an adjuvant, and its specific steps are as follows:
[0034] (1) Antigen preparation: Dilute Muscovy duck hepatitis virus GS14 strain 8000 times with normal saline, inoculate 12-day-old susceptible Muscovy duck embryos through the allantoic cavity, 0.15ml / embryo, collect and inoculate dead Muscovy ducks within 60-168 hours Embryo allantoic fluid, if the sterility test is qualified, is the antigen, and then stored at -30°C;
[0035] (2) Antigen inactivation: the antigen in step (1) is added into a formaldehyde solution with a final volume concentration of 0.1%, and inactivated at 37°C for 24 hours, during which the antigen is shaken 4 times for 3 minutes each time; the formaldehyde solution The content of formaldehyde in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com