Crystal form D of anisodine hydrobromide as well as preparation method and use of crystal form D
A kind of technology of anisodine hydrobromide and crystal form, applied in the crystal form D of anisodine hydrobromide and the field of preparation and use thereof, can solve the problem that no patent discloses the preparation method of anisodine hydrobromide crystal form, etc. problem, to achieve the effects of low cost, good stability, and simple operation of the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~2
[0055] Anti-solvent addition method to prepare anisodine hydrobromide crystal form D:
[0056] Weigh a certain mass of anisodine hydrobromide solid, dissolve it in a certain volume of positive solvent in Table 1, and add anti-solvent dropwise. After adding the anti-solvent, solids were precipitated, and the stirring was continued until a large amount of solids were precipitated, and the solids were collected by centrifugation and dried. The solids obtained in the examples in Table 1 are respectively marked as samples 1~2, and after testing, samples 1~2 are all crystal form D of anisodine hydrobromide.
[0057] Table 1
[0058] serial number
Embodiment 3~6
[0060] Preparation of anisodine hydrobromide crystal form D by gas-liquid infiltration method:
[0061] Weigh a certain mass of anisodine hydrobromide solid, dissolve it in a certain volume of positive solvent in Table 2, and place it in a 3mL vial, add 3mL of anti-solvent to the 20mL vial, place the above-mentioned 3mL vial In the above 20 mL vial, the 20 mL vial was sealed and allowed to stand at room temperature for 4 days. If a solid precipitated, centrifuge and dry; if no solid precipitated, the solution was evaporated at room temperature. The solids were collected, and the solids obtained in the examples in Table 2 were marked as samples 3-6 respectively. After testing, samples 3-6 were all crystal form D of anisodine hydrobromide.
[0062] Table 2
[0063] serial number
Embodiment 7
[0065] The preparation method of anisodine hydrobromide crystal form D:
[0066] Weigh 14.7mg of anisodine hydrobromide, heat to 165°C and cool to room temperature. The solid was collected, and it was detected that the obtained solid was Form D.
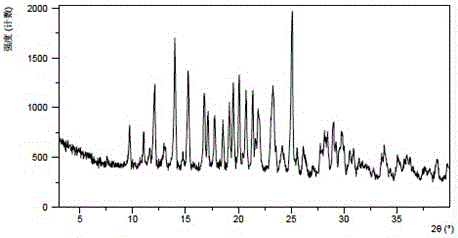
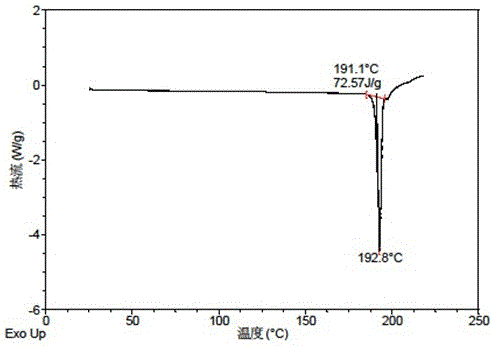
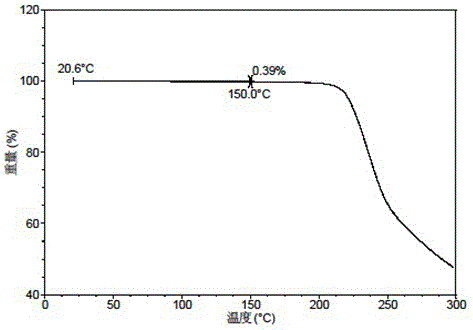
[0067] The X-ray powder diffraction data of the crystal forms obtained in this example are shown in Table 3. Its XRPD pattern is as follows figure 1 , and its DSC graph is shown in figure 2 , and its TGA figure is shown in image 3 .
[0068] table 3
[0069] 2theta
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com