A kind of inactivated vaccine of mycoplasma synovial bursa
A technology of mycoplasma synovialis and inactivated vaccine, which is applied in the directions of vaccines, veterinary vaccines, and antibody medical components to achieve the effects of preventing epidemics, making up for market vacancies, and reducing morbidity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, the screening of bacterial strain
[0022] 1 Culture medium for the production of Mycoplasma synovialis
[0023] 1.1 Preparation of improved CM liquid medium Take 21.0g of PPLO broth, 5g of glucose, add to 1000ml of deionized water, and 1ml of 1% phenol red solution. Add 200ml of inactivated horse serum, 200ml of 25% yeast extract, 1.2ml of 800,000 units / ml penicillin solution, 30ml of 1% coenzyme I solution, 30ml of 1% L‐cysteine solution, and use 1mol / L sodium hydroxide Adjust the pH value of the solution to 7.6-7.8, and store at 2-8°C.
[0024] 1.2 Preparation of improved CM solid medium Take 21.0g of PPLO broth, 5g of glucose, 15.0g of agar powder and add deionized water to 1000ml, mix the above ingredients and heat to dissolve, quantitatively dispense, sterilize at 116°C for 30 minutes, put in 2 Store at ~8°C. Heat and dissolve 100ml of solid medium before use. When the temperature drops to about 60°C, add 20.0ml of inactivated horse serum, 20ml ...
Embodiment 2
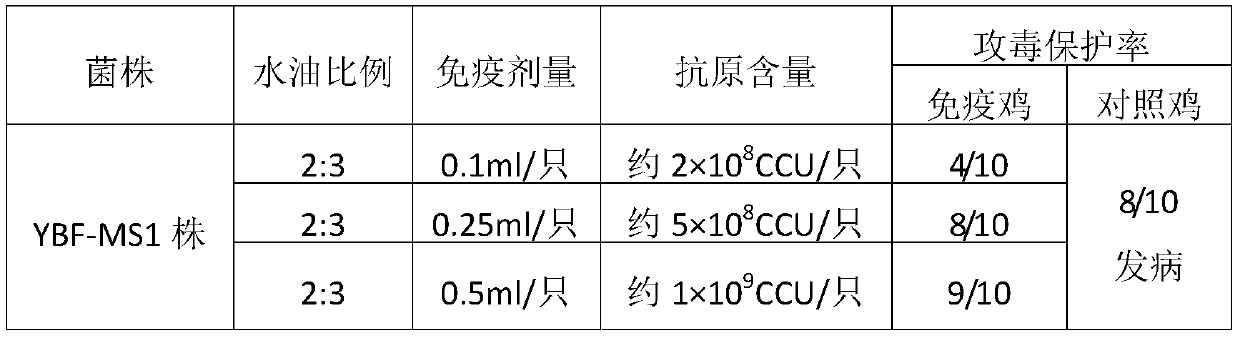
[0052] Embodiment 2: production method of mycoplasma synovial bursa inactivated vaccine
[0053] 1 material
[0054] 1.1 Strain The Mycoplasma gallinarum YBF-MS1 strain was used to prepare the vaccine.
[0055] 1.2 Medium The improved CM liquid medium was provided by Qingdao Yibang Bioengineering Co., Ltd.
[0056] 1.3 Detection reagents Plate agglutination antigen, positive serum and negative serum were all provided by Qingdao Yibang Bioengineering Co., Ltd.
[0057] 1.4SPF chickens were purchased from Beijing Meria Weitong Experimental Animal Technology Co., Ltd.
[0058] 1.5 shearing machine T25 type, produced by German IKA company.
[0059] 1.6 High-speed refrigerated continuous flow centrifuge GR22GIII, purchased from Hitachi, Japan.
[0060] 2 methods
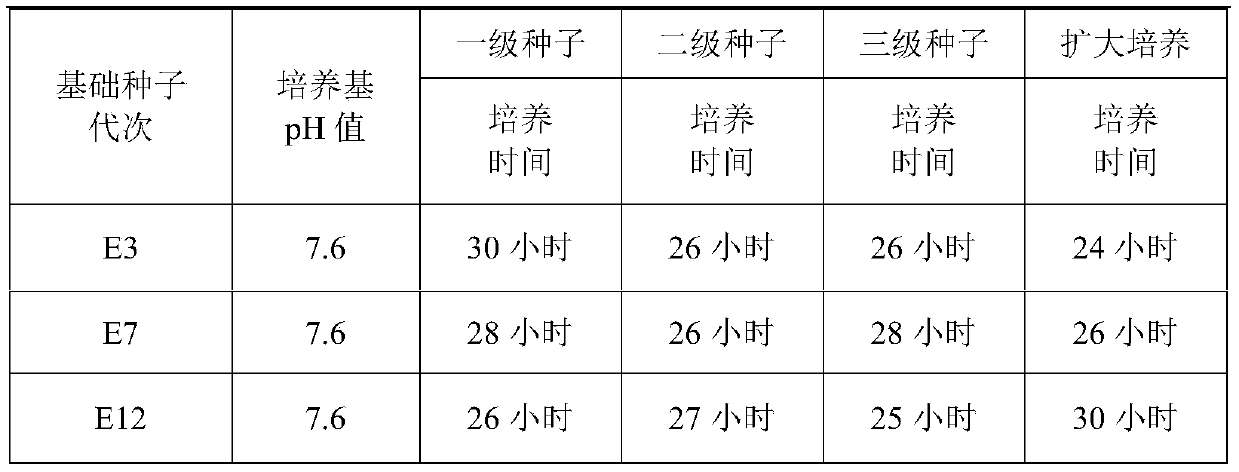
[0061] 2.1 Preparation of seeds for production
[0062] 2.1.1 Primary seed propagation Reconstitute the freeze-dried seeds of E3, E7, and E12 generations of Mycoplasma gallinarum YBF-MS1 strain with improved CM liqu...
Embodiment 3
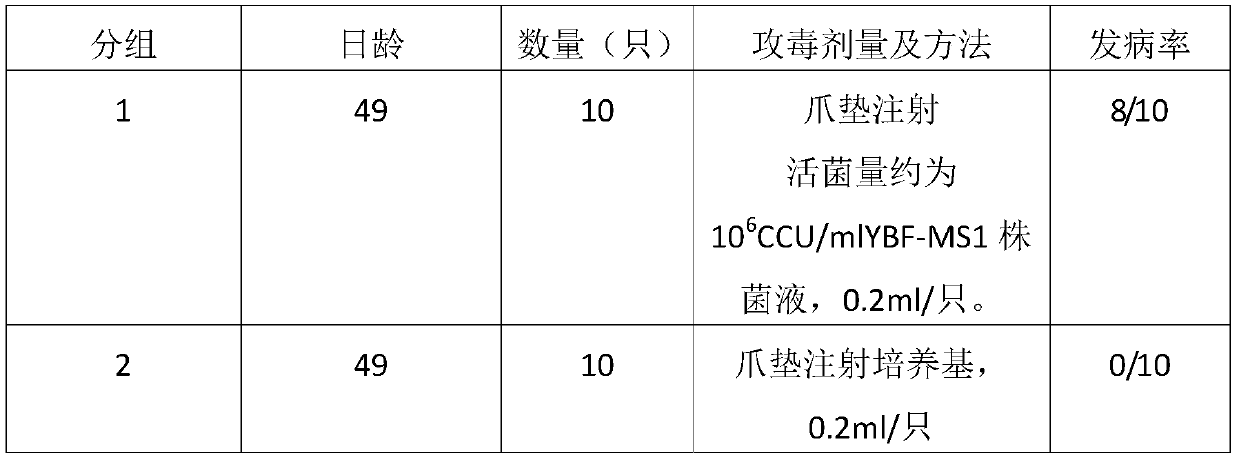
[0129] Embodiment 3, the mycoplasma synovialis inactivated vaccine (YBF-MS1 strain) prepared by the present invention single-dose inoculation, single-dose repeated inoculation and once over-dose inoculation safety test:
[0130] 1 material
[0131] 1.1 The 21-day-old and 200-day-old SPF chickens used in the experiment were all purchased from Beijing Merial Weitong Experimental Animal Technology Co., Ltd., and were repurchased one week in advance and then isolated in the experimental animal room of Qingdao Yibang Bioengineering Co., Ltd. under negative pressure Feed in the container and use after observing no abnormalities.
[0132] 1.2 Vaccine Mycoplasma synovialis inactivated vaccine (YBF-MS1 strain), trial-produced by the laboratory, batch numbers are: 1201, 1202, 1203.
[0133] 2 methods
[0134] 2.1 Single-dose vaccination safety test Take 21-day-old SPF chickens and divide them into 2 groups, 10 in each group. Group 1 was subcutaneously injected with 1201 batches of in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com