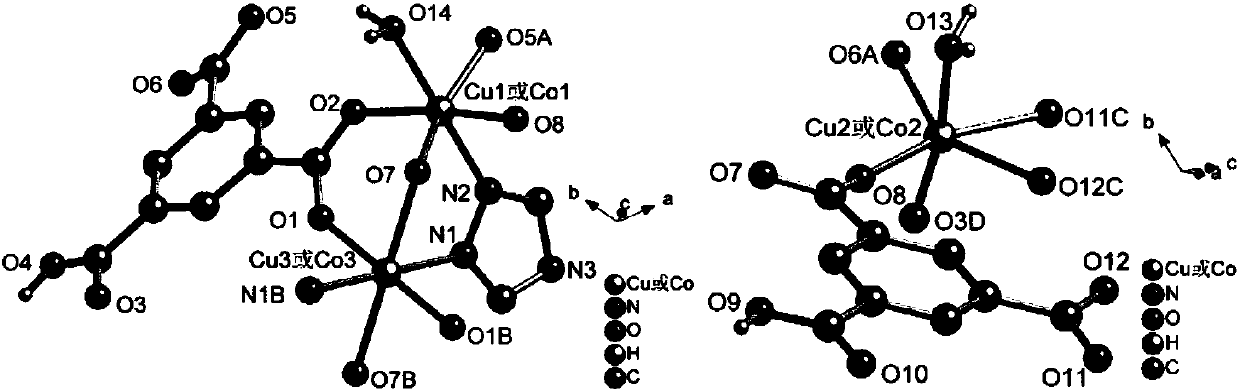
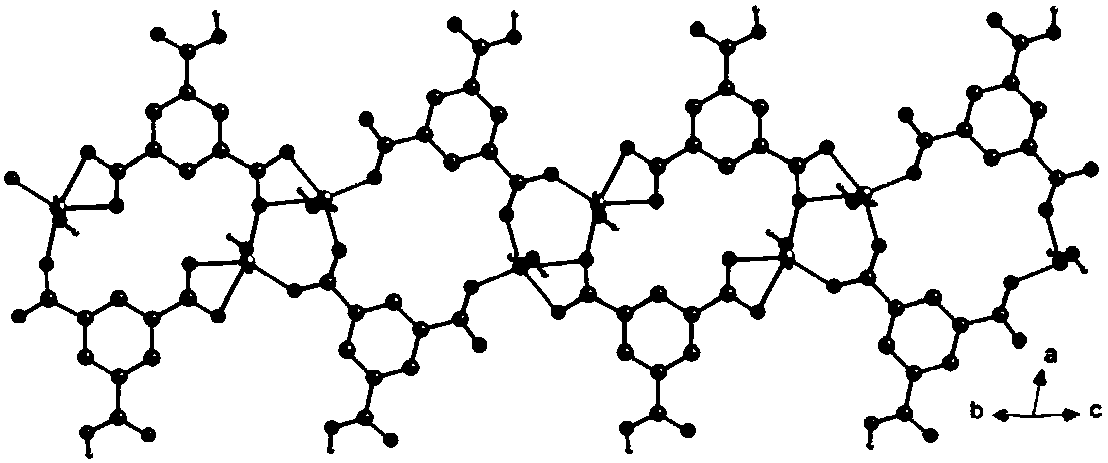
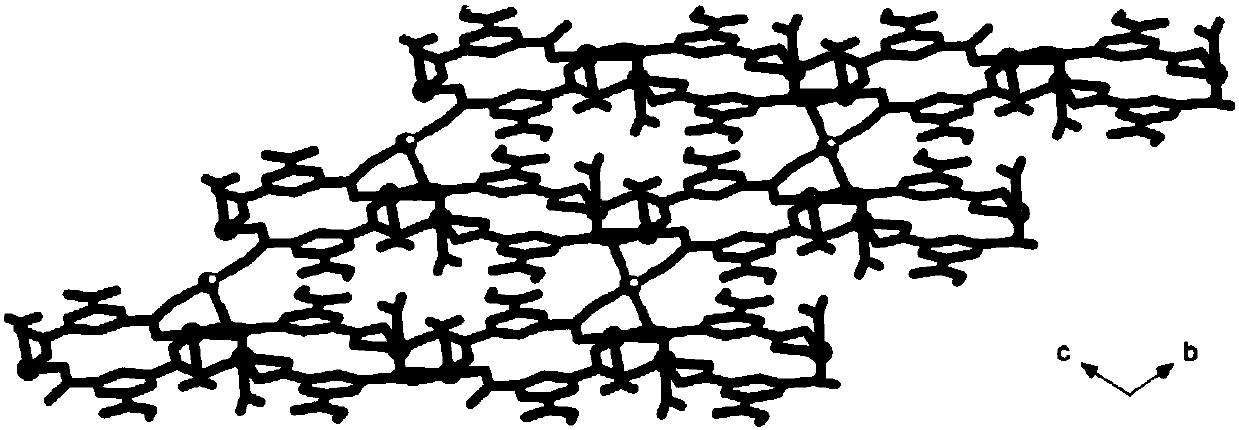
A kind of cu(ii)-co(ii) mixed metal coordination polymer, its preparation method and application
A technology of coordination polymers and mixed metals, applied in the field of Cu-Co mixed metal complexes, to achieve the effect of easy operation, simple preparation method and controllable conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Trimellitic acid (0.5mmol, 105.1mg), 1,2,4-triazole (0.2mmol, 13.8mg), ammonium formate (0.2mmol, 12.6mg), lithium carbonate (0.1mmol, 7.4mg), Copper nitrate (0.4mmol, 96.6mg) and cobalt nitrate (0.4mmol, 116.4mg) were dissolved in water (6mL), and sealed in a 25mL hydrothermal reaction kettle. Then heat the reaction mixture at 10°C per hour to 160°C, maintain this temperature for 3 days, and then lower it to room temperature to obtain purple columnar crystals, which are the target product Cu(II)-Co(II) mixed metal coordination polymerization The yield is about 51%. The main infrared absorption peaks of the target product are: 3602w, 1705m, 1613s, 1566s, 1442s, 1388vs, 1268w, 1168w, 1098m, 1005w, 884w, 750m, 666m, 539w, 448w.
Embodiment 2
[0046] Trimellitic acid (0.3mmol, 63.0mg), 1,2,4-triazole (0.06mmol, 4.1mg), ammonium formate (0.06mmol, 3.8mg), lithium carbonate (0.06mmol, 4.4mg), Copper nitrate (0.24mmol, 58.0mg) and cobalt nitrate (0.24mmol, 70.0mg) were dissolved in water (6mL), and sealed in a 25mL hydrothermal reaction kettle. Then the reaction mixture was heated at 10°C per hour to 140°C, maintained at this temperature for 3 days, and then lowered to room temperature to obtain purple columnar crystals, the target product, with a yield of about 42%. The main infrared absorption peaks of the target product are: 3600w, 1703m, 1610s, 1562s, 1440s, 1385vs, 1262w, 1163w, 1092m, 998w, 880w, 743m, 662m, 531w, 442w.
Embodiment 3
[0048] Trimellitic acid (3mmol, 630.4mg), 1,2,4-triazole (0.6mmol, 41.4mg), ammonium formate (0.6mmol, 37.8mg), lithium carbonate (0.6mmol, 44.3mg), nitric acid Copper (2.4mmol, 580.0mg) and cobalt nitrate (2.4mmol, 698.5mg) were dissolved in water (6mL), and sealed in a 25mL hydrothermal reaction kettle. Then the reaction mixture was heated at 10°C per hour to 180°C, maintained at this temperature for 3 days, and then lowered to room temperature to obtain purple columnar crystals, the target product, with a yield of about 27%. The main infrared absorption peaks of the target product are: 3610w, 1709m, 1617s, 1555s, 1447s, 1380vs, 1267w, 1160w, 1089m, 992w, 887w, 740m, 661m, 536w, 440w.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com