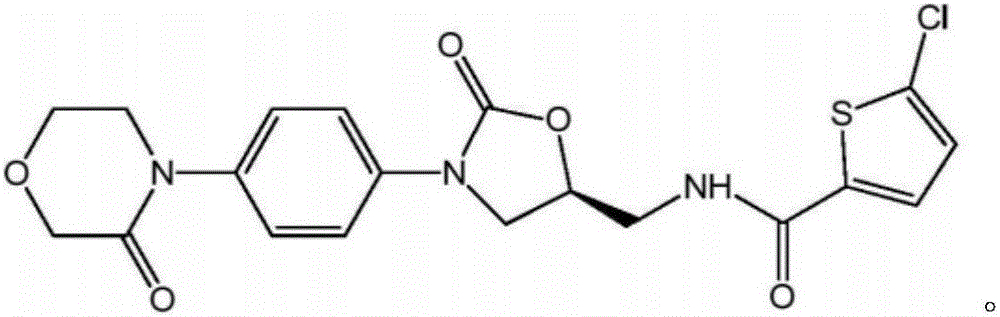
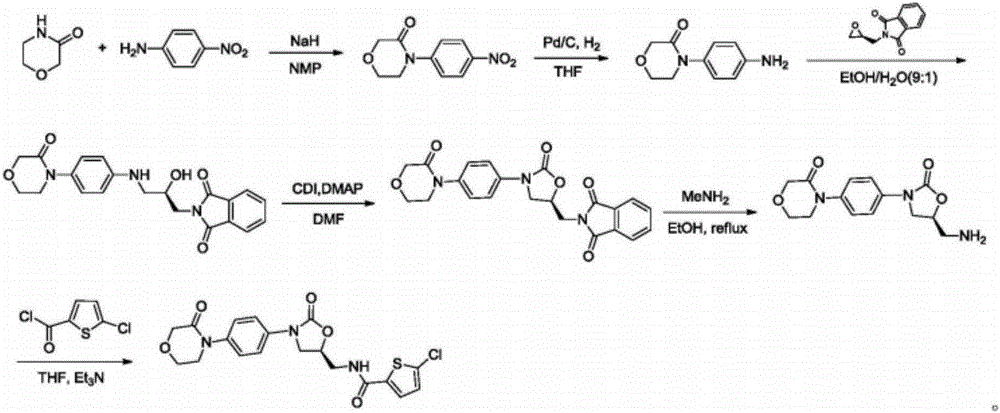
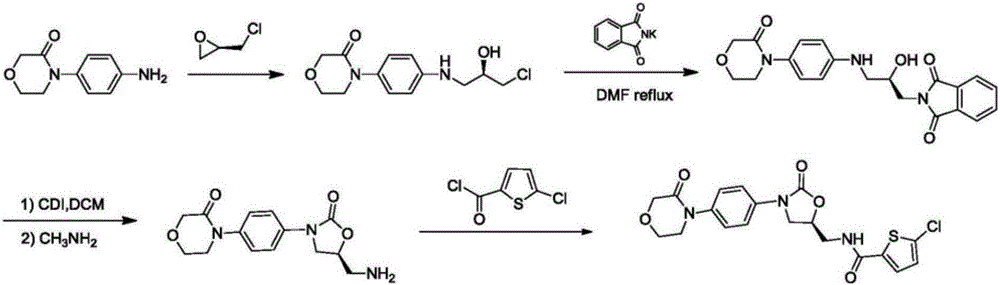
Preparation method of rivaroxaban
A technology of rivaroxaban and compounds, applied in the field of medicine and chemical industry, can solve the problems of unavailable intermediates, high price, large amount of reagents, etc., and achieve the effects of efficient preparation, easy handling, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0059] Embodiment 1-1: Synthesis of Compound 2
[0060] Add phthalimide potassium salt (55.6g, 0.3mol), (S)-4-chloro-3-hydroxybutyronitrile (compound 1, 35.9g, 0.3mol) and 300mL DMF into the reaction flask, heat to 80 After reacting at ℃ for 3 h, the reaction solution was poured into 400 mL of water, stirred for 10 min, filtered with suction, and dried under reduced pressure to obtain 63.7 g of white solid (Compound 2), with a yield of 92.3%.
Embodiment 1-2
[0061] Embodiment 1-2: the synthesis of compound 2
[0062] Add phthalimide potassium salt (66.7g, 0.36mol), (S)-4-chloro-3-hydroxybutyronitrile (compound 1, 35.9g, 0.3mol) and 300mL DMF into the reaction flask, heat to 70 After reacting at ℃ for 4 h, the reaction solution was poured into 400 mL of water, stirred for 10 min, filtered with suction, and dried under reduced pressure to obtain 66.9 g of white solid (Compound 2), with a yield of 96.8%.
Embodiment 2-1
[0063] Embodiment 2-1: Synthesis of Compound 3
[0064] Under magnetic stirring, the compound 2 (57.6g, 0.25mol) prepared in Example 1-1 was added into dichloromethane (300mL) and cooled in an ice bath, 30% hydrogen peroxide (110mL) was added, tetrabutylsulfuric acid Ammonium hydrogen (17 g, 50 mmol), and 20% aqueous sodium hydroxide (100 mL). The reaction mixture was stirred and heated to room temperature. After 1.5 hours, dichloromethane was added, the organic layer was separated, washed with brine, the organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was decompressed to remove the solvent to obtain white solid compound 3 (60.7g) , the HPLC purity was 99.5%, and the yield was 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com