A kind of highly active bifunctional catalyst and its preparation method and application
A dual-functional catalyst and high-activity technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of reduced reaction rate, difficult chiral cyclic carbonic Ester synthesis and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] Compound b: In a 100mL round-bottomed flask, compound 1 (3.60g, 10.0mmol) and anhydrous potassium carbonate (1.5g, 12.0mmol) were dissolved in 50mL refined acetonitrile, and refined diethylamine (1.7mL, 12.0mmol) was added, Reacted at 80°C for 24 h, followed the reaction by TLC until no raw materials remained, stopped the reaction, cooled the reaction liquid to room temperature, filtered, and removed the solvent under reduced pressure to obtain the crude product as a white turbid liquid. Dissolve it in 10ml of ethyl acetate, add 40mL of 2mol / L dilute hydrochloric acid solution, and stir vigorously for 0.5h. The aqueous phase was separated, the organic phase was extracted with water (20 mL×3), the aqueous phases were combined, and saturated sodium bicarbonate solution was slowly added to make it alkaline to pH=8, and extracted with dichloromethane (50 mL×3). The combined organic phases were washed with saturated brine (200 mL×1), dried over anhydrous sodium s...
Embodiment 2
[0074]
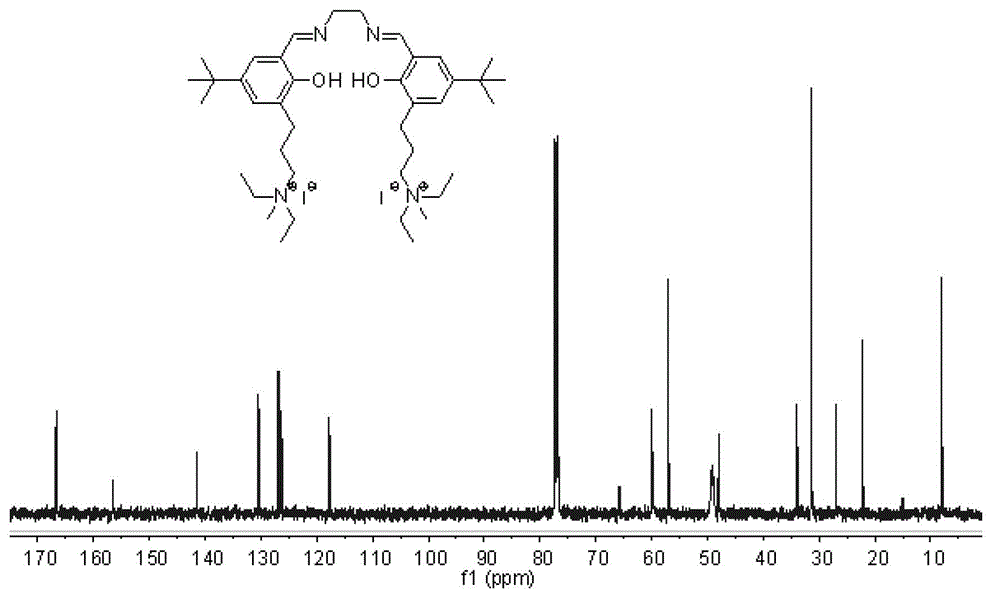
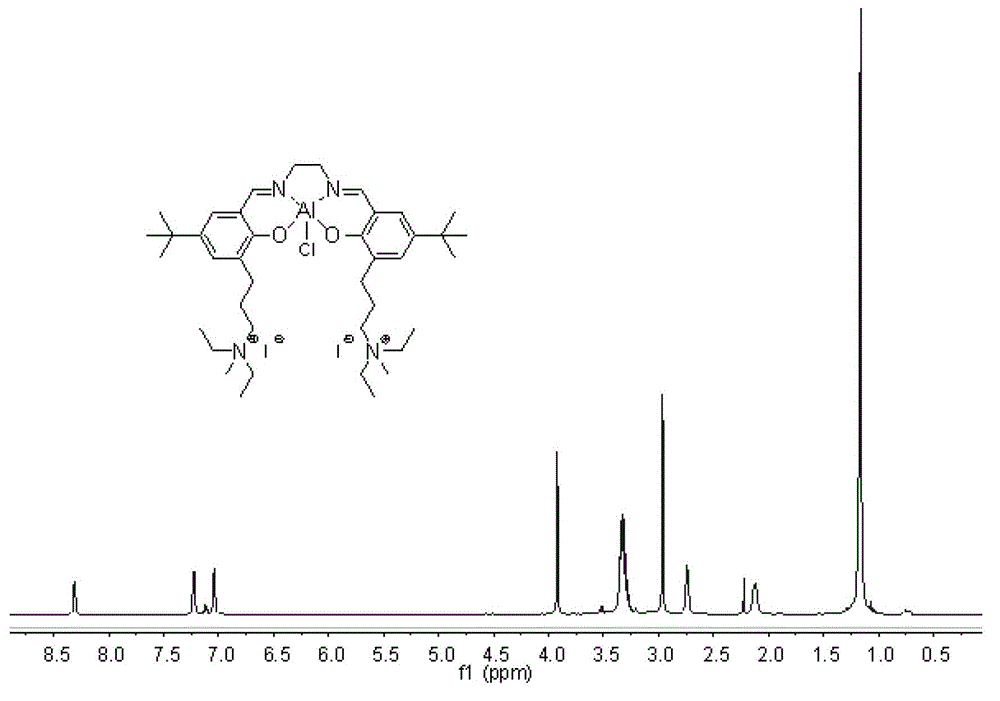
[0075] Compound g: In a 100mL round bottom flask, ethylenediamine monohydrochloride (0.19g, 2.0mmol) and 3,5-di-tert-butyl salicylaldehyde (0.47g, 2.0mmol) were dissolved in 30mL of anhydrous methanol, join in Molecular sieve. After reacting at 20°C for 2 h, refined triethylamine (0.27 mL, 2.0 mmol) and compound d (0.87 g, 2.0 mmol) were added, and 30 mL of ethanol was added, and stirring was continued for 4 h. Stop the reaction, filter with suction, wash the filter cake with dichloromethane, and remove the solvent from the filtrate under reduced pressure to obtain a crude product. Separation and purification by column chromatography (silica gel column; developer volume ratio: petroleum ether / ethyl acetate / triethylamine=100 / 10 / 1) to obtain compound g as a yellow solid (yield: 0.49g, yield: 38 %).
[0076] 'H NMR (400MHz, CDCl 3 )δ13.51(s,1H),13.36(s,1H),8.35(s,1H),8.32(s,1H),7.29(s,1H),7.19(s,1H),7.07(s,1H ),7.01(s,1H),3.84–3.89(m,4H),3.46–3.49(m,4H),3.32–3.3...
Embodiment 3
[0084] In a stainless steel autoclave with a volume of 200mL, add it in the following order at ambient temperature: the catalyst is dissolved in alkylene oxide, the system is heated to the set temperature, and then carbon dioxide gas is fed to the set pressure (maintained during the reaction). Constant pressure), after reacting for a certain period of time under stirring, the unreacted carbon dioxide in the reactor was slowly released, and the residue was analyzed by infrared spectrum and nuclear magnetic spectrum, and no by-products such as polycarbonate and polyether were found. The cyclic carbonate was distilled off under reduced pressure, and the purity of the product was determined by gas phase or liquid chromatography and nuclear magnetic resonance spectroscopy. It was found that the selectivity of the cyclic carbonate in all systems was higher than 99.5%. See Table 1 to Table 6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com