A preparation method of pyridine-wrapped lead cadmium sulfur nanoparticles and products thereof
A nanoparticle, lead cadmium sulfur technology, applied in nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve problems such as inability to perform solution treatment, complex synthesis process requirements, and not suitable, etc. To achieve the effects of non-toxicity, good application prospects and process control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
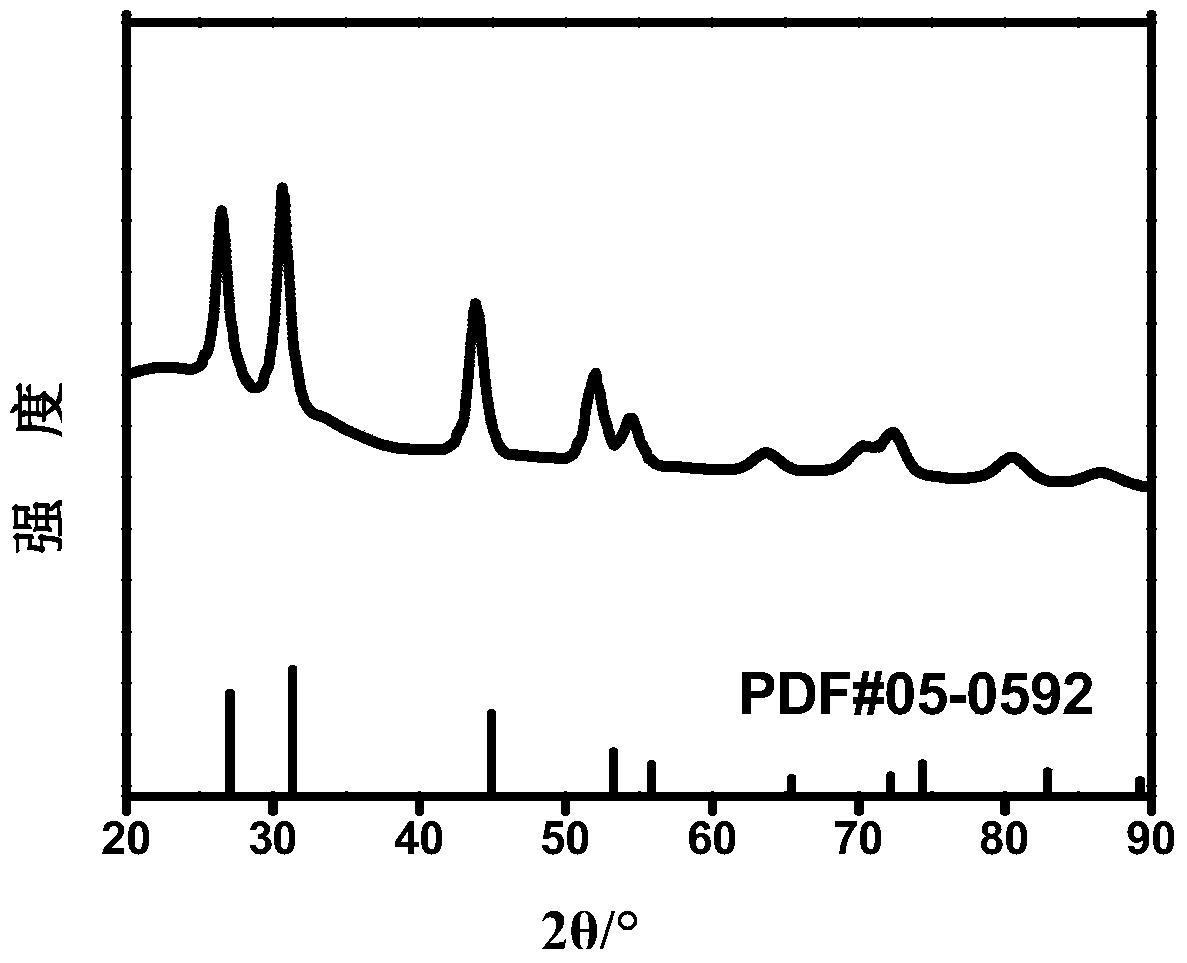
[0031] Dissolve 0.7mmol lead acetate and 0.3mmol cadmium acetate in a mixed solvent of 40mL pyridine and 10mL water, and heat up to 80°C; at the same time, dissolve 1mmol sodium sulfide in 20mL water; then, drop the sodium sulfide solution into the metal source solution for 20min After dropping, the reaction temperature was maintained at 80°C for 6h. The lead-cadmium-sulfur nanoparticles were settled down with acetone and centrifuged to obtain a black solid. Add 30mL of water, sonicate for 10min, then add 30mL of acetone, and centrifuge to obtain pure pyridine-wrapped lead, cadmium, and sulfur nanoparticles. Its XRD pattern is as follows figure 1 shown. The synthesized pyridine-wrapped lead-cadmium-sulfur nanoparticles are in the cubic phase, which is consistent with the XRD standard card JCPDSNo.65-5915. figure 2 The scanning electron microscope image of the pyridine-wrapped lead-cadmium-sulfur nanoparticles prepared for this example shows that the bright particles in the...
Embodiment 2
[0033] Dissolve 0.7mmol lead chloride and 0.3mmol cadmium acetate in a mixed solvent of 40mL pyridine and 10mL water, and heat up to 80°C; at the same time, dissolve 1mmol sodium sulfide in 20mL methanol; then, drop the sodium sulfide solution into the metal source The solution was dripped in 20 minutes; the reaction temperature was maintained at 80°C, and the reaction was carried out for 1 hour. The lead-cadmium-sulfur nanoparticles were settled down with acetone and centrifuged to obtain a black solid. Add 30mL of water, sonicate for 10min, then add 30mL of acetone, and centrifuge to obtain pure pyridine-wrapped lead, cadmium, and sulfur nanoparticles.
Embodiment 3
[0035] Dissolve 0.7mmol lead acetate and 0.3mmol cadmium chloride in a mixed solvent of 40mL pyridine and 10mL water, and heat up to 80°C; at the same time, dissolve 1mmol sodium sulfide in 20mL ethanol; then, drop the sodium sulfide solution into the metal source solution , 20min dripping; maintain the reaction temperature 80 ℃, the reaction 6h. The lead-cadmium-sulfur nanoparticles were settled down with acetone and centrifuged to obtain a black solid. Add 30mL of water, sonicate for 10min, then add 30mL of acetone, and centrifuge to obtain pure pyridine-wrapped lead, cadmium, and sulfur nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com