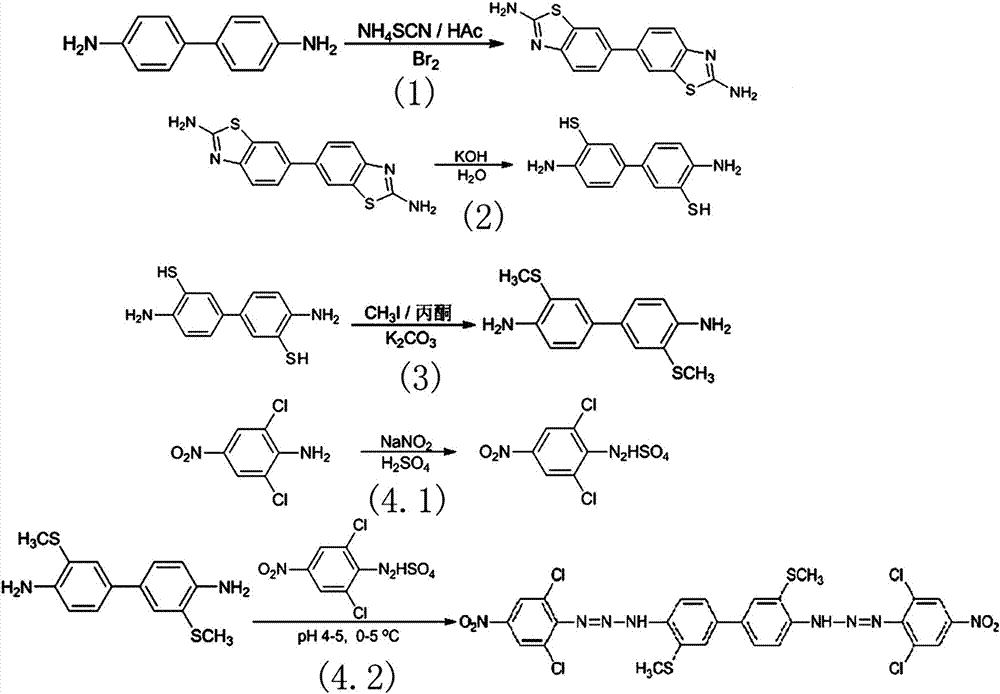
3,3'-dimethylthio-4,4'-bis(2,6-dichloro-4-nitrophenyldiazoamino)biphenyl and preparation method and application
A technology of diaminobiphenyl dithiazole and dimethylthio, which is applied in the field of bistriazene compounds, can solve the problems of difficult heavy metal ion fluorescence detection, large interference of coexisting ions, unsatisfactory sensitivity and selectivity, etc., and achieves selectivity. Good, improve sensitivity, enhance the effect of π electron mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the 3,3'-dimethylthio-4,4'-bis(2,6-dichloro-4-nitrophenyldiazoamino)biphenyl comprises the following steps:
[0028] (1) Preparation of 2,2'-diaminobiphenylbithiazole: Add 18.4 g of 4,4'-diaminobiphenyl to a 500 ml three-necked flask equipped with a drying tube, condenser and dropping funnel (0.1 mol), 150 ml of glacial acetic acid and 79.6 g of potassium thiocyanate, stirred at room temperature for 20 min, then measured 9.0 ml of bromine dissolved in 120 ml of glacial acetic acid, slowly added dropwise to the reaction mixture, and kept the reaction temperature at 60°C , continue to stir and react for 22 h, pour the mixture in the three-necked flask into cold water, add ammonia to it under constant stirring, adjust the pH value to 8, let it stand, and filter and dry the solution after cooling; V 乙酸乙酯 : V 乙醇 = 1:1 mixed solution of ethyl acetate and ethanol was recrystallized, dried under vacuum at 60°C to obtain 24 g of off-white solid 2,2'...
experiment example 1
[0034] Experimental example 1: DMSDCNPDP on Hg 2+ photometric analysis.
[0035] Add the standard solution of ≤20 μg Hg (Ⅱ) to the 25 ml volumetric flask in turn, add 3.0 ml of borax-sodium hydroxide buffer solution with pH 10.0, 2.0 ml of 30 g / L TritonX-100 solution, shake well, add 0.2 g / LDMSDCNPDP DMF solution 2.0 ml, diluted with water to the mark, using the reagent blank as a reference, using a 1cm cuvette, at 560nm, measure the absorbance of the complex solution.
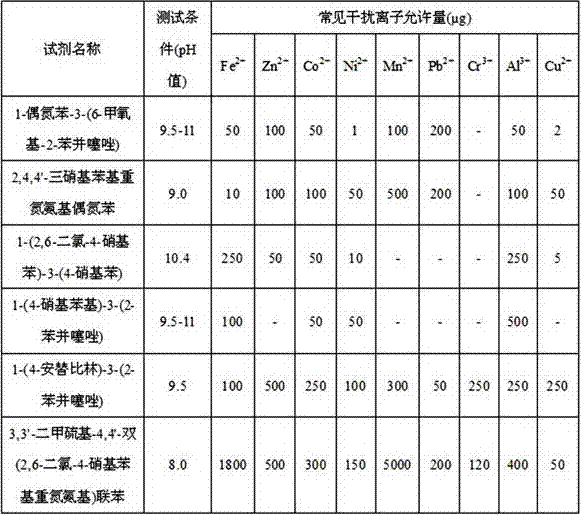
[0036] Treat the hair and water samples according to the method in the literature [Analytical Chemistry, Guo Zhongxian, 1996, Volume 24, No. 1, Page 65-68], add 1ml of mixed masking agent, and measure according to the test method. The results are shown in Table 1. The comparison results of the sensitivity of the present invention to mercury and the sensitivity of existing reagents to mercury are shown in Table 2, and the results show that the compound of the present invention has high detection sensitivity fo...
experiment example 2
[0042] Experimental example 2: Determination of lead (II) by DMSDCNPDP fluorescence quenching method: in a 25 ml volumetric flask, add 1.2×10 lead ions in sequence -9 ~1.6×10 -7 mol / L lead (Ⅱ) solution, 3 ml 3% Tween-80 aqueous solution, 2.0 ml pH8.2 borax-sodium hydroxide buffer solution, 2.5 ml 2×10 -5 mol / L DMSDCNPDP solution, then double-distilled water to volume. Using a 1cm cuvette, at the excitation wavelength (λ ex ) 246 nm, emission wavelength (λ em ) at 440 nm to measure its fluorescence intensity, and record ΔF (the fluorescence absorbance of reagent blank is 100).
[0043] Process the sample according to the literature [Physical and Chemical Test-Chemistry Volume, Wei Qin, Shou Chongqi, etc., 2004, Volume 40, No. 1, Page 12-14], and then use the experimental method to determine. The results are shown in Table 4. The results show that the reagent fluorescence quenching method of the invention is better in sensitivity and selectivity than the existing reagents ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com