Method for determining rivaroxaban and impurities thereof through adopting liquid chromatography
A technology of rivaroxaban and liquid chromatography, which is applied in the field of determination of impurities in rivaroxaban and its preparations by liquid chromatography, can solve problems such as the inability to realize effective quality control of rivaroxaban and its preparations, and achieve guaranteed The effect of quality control, improved resolution, and peak shape symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Instruments and Conditions
[0061] Agilent1260 liquid chromatograph and chemical workstation; automatic sample injection; Kromasil100-5C18 column (5mm, 250×4.6mm) as separation column; UV detector wavelength: 250nm; mobile phase: 0.05% trifluoroacetic acid and 0.01 mol / L sodium dihydrogen phosphate mixed solution (adjust pH value to 4.50 with sodium hydroxide solution) as mobile phase A, acetonitrile as mobile phase B, gradient elution; 0 minutes, mobile phase A is 93% (V / V ), the mobile phase B is 7% (V / V); from 0 minutes to 9 minutes, the mobile phase A linearly decreases to 70% (V / V), and the mobile phase B linearly increases to 30% (V / V); 9 minutes From 28 minutes to 28 minutes, the mobile phase A linearly decreased to 60% (V / V), and the mobile phase B linearly increased to 40% (V / V); from 28 minutes to 50 minutes, the mobile phase A linearly decreased to 30% (V / V ), the mobile phase B increased linearly to 70% (V / V); 50.1 minutes, the mobile phase A was 93% (V / V)...
Embodiment 2
[0068] Determination of impurities in raw materials of rivaroxaban.
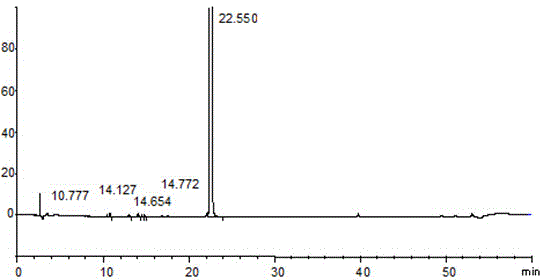
[0069] Take about 12.5mg of rivaroxaban, weigh it accurately, put it in a 25ml measuring bottle, add diluent (methanol: acetonitrile: water = 2:1:1V / V) and sonicate to dissolve and dilute to the mark, shake well, and use as Need testing solution; Precision measures need testing solution appropriate amount in addition, adds diluent and makes concentration and is the solution of need testing solution concentration 1.0%, as contrast solution; Carry out liquid chromatography analysis according to the chromatographic condition of embodiment 1, for If there are impurity peaks (except solvent peaks) in the chromatogram of the test solution, the peak area of the known impurities multiplied by the correction factor shall not be greater than 1 / 2 (0.5%) of the main peak area of the control solution, and the peak area of a single impurity shall not be greater than that of the control The main peak area of the so...
Embodiment 3
[0071] Determination of impurities in rivaroxaban tablets by liquid chromatography.
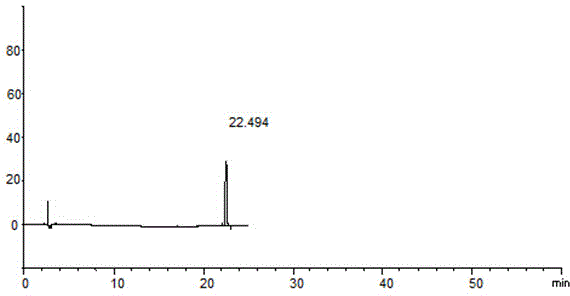
[0072] Take an appropriate amount of this product (approximately equivalent to rivaroxaban 12.5mg), put it in a 25ml measuring bottle, add a diluent (ethyl methanol: acetonitrile: water = 2:1:1V / V) and sonicate to dissolve and dilute to the mark. Shake well, as the test solution; in addition, accurately measure the appropriate amount of the test solution, add a diluent to make a solution with a concentration of 1.0% of the test solution, and use it as a control solution; Need testing solution identical method prepares blank adjuvant need testing solution; Carry out liquid chromatography analysis according to the chromatographic condition of embodiment 1, if impurity peak (except solvent peak and blank adjuvant peak) is arranged in the chromatogram of need testing solution, The peak area of the known impurities multiplied by the correction factor shall not be greater than 1 / 2 (0.5%) of the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com