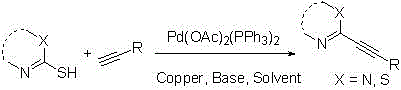Method for preparing C2-position alkynyl compound by catalyzing thiamine type compound desulfuration through cuprous halides
A compound and thiamine technology, which is applied in the field of cuprous halide catalyzing the desulfurization of thiamine compounds to prepare C2-position alkynyl compounds, can solve the problems of cumbersome experimental steps, harsh conditions, environmental pollution, etc. Simple, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 28 mg (0.2 mmol) of 2-mercapto-5-pyrimidinecarbaldehyde, 33 mg (0.4 mmol) of 3,3-dimethyl-1-butyne and 1.5 mg (2%) of palladium triphenylphosphine acetate, 102 mg ( 1mmol) triethylamine, 2ml N,N-dimethylformamide were added to the Schlenk tube under nitrogen atmosphere, and copper salt (0.6mmol) (cuprous iodide, cuprous chloride, cuprous bromide , cuprous oxide, copper acetate monohydrate), at 110 o The reaction was stirred at C for 1 hour. Through GC detection and analysis, when cuprous iodide is used as a desulfurizing agent, the yield of the coupling reaction can reach 60% yield.
Embodiment 2
[0025] 28 mg (0.2 mmol) of 2-mercapto-5-pyrimidinecarbaldehyde, 33 mg (0.4 mmol) of 3,3-dimethyl-1-butyne and 1.5 mg (2%) of palladium triphenylphosphine acetate, 114 mg ( 0.6mmol) cuprous iodide and 1.0mmol base (triethylamine, sodium carbonate, pyridine, sodium acetate, N,N-diisopropylethylamine, 1,8-diazabicycloundec-7- Alkenes) were added to the Schlenk tube under nitrogen atmosphere, under N 2 Add 2.0ml of N,N-dimethylformamide under the environment, at 110 o The reaction was stirred at C for 12 hours. Through GC detection and analysis, under the catalysis of 5 times the equivalent of sodium carbonate, the coupling reaction can reach a yield of 92%.
Embodiment 3
[0027] 28 mg (0.2 mmol) of 2-mercapto-5-pyrimidinecarbaldehyde, 33 mg (0.4 mmol) of 3,3-dimethyl-1-butyne and 1.5 mg (2%) of palladium triphenylphosphine acetate, 114 mg ( Add 0.6mmol) cuprous iodide, 106mg sodium carbonate into the Schlenk tube under nitrogen atmosphere, add 2.0ml solvent (N,N-dimethylformamide, toluene, tetrahydrofuran, acetonitrile, 1,4-di Oxyhexane), at 110 o The reaction was stirred at C for 1 hour. Through GC detection and analysis, under the condition of N,N-dimethylformamide as solvent, the coupling reaction can reach a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com