Preparation method of ZrO2 nanosheet supported ruthenium catalyst
A ruthenium catalyst and nanosheet technology, which is applied in the field of preparation of ZrO2 nanosheet-supported ruthenium catalysts, can solve the problems of large amount of non-supported precious metal Ru, agglomeration and deactivation, etc., and achieve the promotion of gas-solid-water-oil four-phase reaction , promote desorption, and enhance the effect of force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
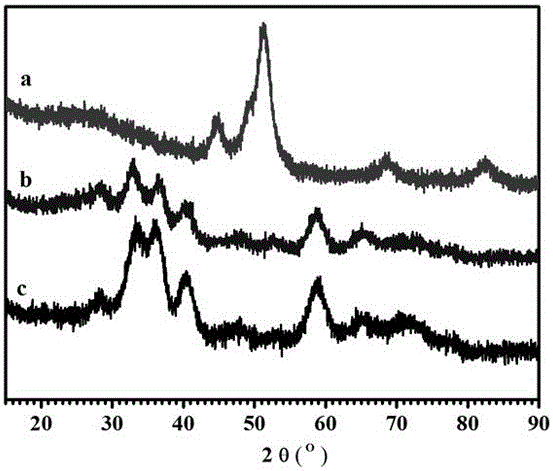
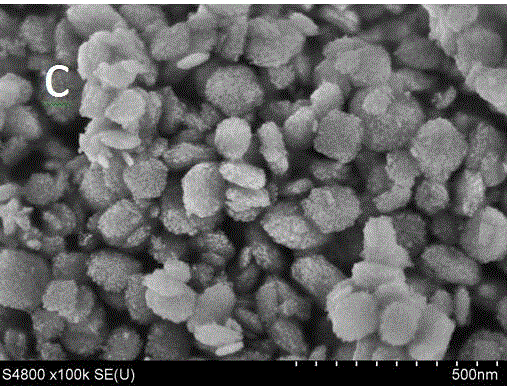
Image
Examples
Embodiment 1
[0028] Weigh 1.3gRuNO (NO 3 ) 3 , 17.1gZrO(NO 3 ) 2 2H 2 O was dissolved in 156mL deionized water, transferred to a hydrothermal kettle with a volume of 200mL, heated at 170°C for 6h, and naturally cooled to room temperature to obtain a khaki-yellow slurry; the slurry was taken out, and 10wt.% NaOH solution was used as a precipitating agent. Precipitate at 60°C, control the pH at the end of the precipitation to be 9, and let it stand for 4 hours; centrifuge and wash the obtained precipitate with deionized water until it is neutral; vacuum dry the precipitate at 80°C for 12 hours, and then store it in a hydrogen atmosphere at 200°C Roasting 6h.
Embodiment 2
[0030] Weigh 0.48gKRuO 4 , 17.1gZrO(NO 3 ) 2 2H 2 O was dissolved in 156mL of 10vol.% alcohol solution, transferred to a 200mL hydrothermal kettle, heated in water at 130°C for 18 hours, and naturally cooled to room temperature to obtain a khaki-yellow slurry; take out the slurry, and use 10wt.% KOH solution as Precipitant, precipitate at 70°C, control the pH of the end of the precipitation to be 13, and let it stand for 12 hours; centrifuge and wash the obtained precipitate with deionized water until neutral; dry the precipitate at 80°C for 12 hours in a hydrogen atmosphere Calcined at 450°C for 4h.
Embodiment 3
[0032] Weigh 2.14gRuCl 3 ·3H 2 O, 20.6gZrOCl 2 ·8H 2 O was dissolved in 150mL deionized water, transferred to a hydrothermal kettle with a volume of 200mL, heated at 150°C for 12h, and naturally cooled to room temperature to obtain a khaki slurry; the slurry was taken out, and 10wt.% NaOH solution was used as a precipitating agent. Precipitate at 80°C, control the pH at the end of the precipitation to 10, let it stand for 12 hours, add 1mL of 30wt.%H 2 o 2 solution; the resulting precipitate was centrifuged and washed with a mixture of acetone and deionized water (volume ratio 1:5) until AgNO 3 The solution was detected to be free of chloride ions; the precipitate was vacuum-dried at 80° C. for 12 hours, and then calcined at 300° C. for 4 hours under a hydrogen atmosphere.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com