Preparation and application of dual-sensitivity amphiphilic polysaccharide-doxorubicin conjugate and pharmaceutical composition thereof
A technology of amphiphilic polysaccharides and doxorubicin, which can be used in drug combinations, medical preparations containing active ingredients, medical preparations containing active ingredients, etc., which can solve poor oral absorption and maintain effective blood drug concentration for a short time , rapid metabolism in the body, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
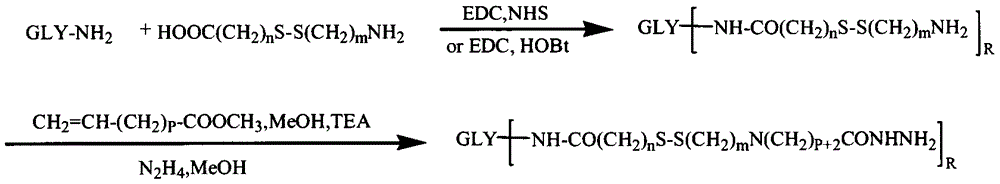
[0053] Example 1: Preparation of Hyaluronic Acid-Cystamine-Adriamycin Conjugate
[0054] 0.1mmol hyaluronic acid, 1mmol cystamine, 0.2mmol EDC and 0.2mmol NHS were dissolved in formamide, and the reaction
[0055] After 24h, use excess acetone to precipitate and filter with suction. The precipitate was reconstituted by adding water, dialyzed with distilled water for 3 days (MWCO=3500), and freeze-dried to obtain a product containing disulfide bonds.
[0056] 0.1 mmol of the product from the previous step, 1 mmol of methyl acrylate and 0.2 mmol of triethylamine were dissolved in an appropriate amount of formamide and methanol mixed solvent, and after 24 hours of reaction, precipitated with excess cold acetone, and filtered with suction. Add water to redissolve the precipitate and dialyze with distilled water for 3 days (MWCO=3500), freeze-dry the obtained product and react it with an appropriate volume of hydrazine hydrate for 24 hours, spin evaporate at 50°C to remove excess ...
Embodiment 2
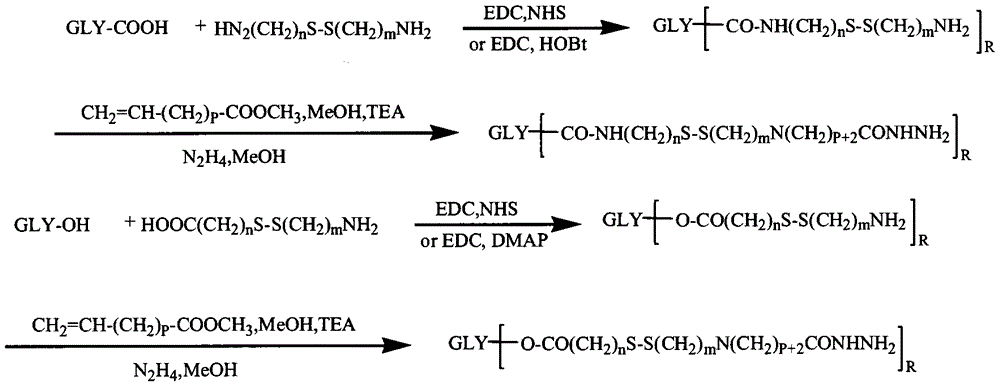
[0058] Example 2: Preparation of Chondroitin Sulfate-Cystamine-Adriamycin Conjugate
[0059] 0.1mmol of chondroitin sulfate, 1mmol of cystamine, 0.4mmol of EDC and 0.4mmol of NHS were dissolved in formamide, reacted for 24h, precipitated with excess acetone, and filtered with suction. The precipitate was reconstituted by adding water, dialyzed with distilled water for 3 days (MWCO=3500), and freeze-dried to obtain a product containing disulfide bonds.
[0060] 0.1 mmol of the product from the previous step, 1 mmol of methyl acrylate and 0.2 mmol of triethylamine were dissolved in an appropriate amount of formamide and methanol mixed solvent, and after 24 hours of reaction, precipitated with excess cold acetone, and filtered with suction. Add water to redissolve the precipitate and dialyze with distilled water for 3 days (MWCO=3500), freeze-dry the obtained product and react it with an appropriate volume of hydrazine hydrate for 24 hours, spin evaporate excess hydrazine hydrate...
Embodiment 3
[0062] Example 3: Preparation of Heparin-Cystamine-Adriamycin Conjugate
[0063] 0.1mmol heparin, 1mmol cystamine, 0.2mmol EDC and 0.2mmol NHS were dissolved in formamide, reacted for 24h, precipitated with excess acetone, and filtered with suction. The precipitate was reconstituted by adding water, dialyzed with distilled water for 3 days (MWCO=3500), and freeze-dried to obtain a product containing disulfide bonds.
[0064] 0.1 mmol of the product from the previous step, 1 mmol of methyl acrylate and 0.2 mmol of triethylamine were dissolved in an appropriate amount of formamide and methanol mixed solvent, and after 24 hours of reaction, precipitated with excess cold acetone, and filtered with suction. Add water to redissolve the precipitate and dialyze with distilled water for 3 days (MWCO=3500), freeze-dry the obtained product and react it with an appropriate volume of hydrazine hydrate for 24 hours, spin evaporate excess hydrazine hydrate at 50°C, dialyze with distilled wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com