Albumin composition highly-carrying cabazitaxel medicine, preparation and preparation method thereof
A cabazitaxel, albumin technology, applied in the direction of drug combination, inactive components of polymer compounds, pharmaceutical formulations, etc., can solve the problem of increasing the amount of albumin injected into the human body, reducing the targeting of nanoparticles, and reducing the targeting of nanoparticles. and other problems, to achieve the effect of being conducive to industrial application, solving toxic and side effects, and being easy to sterilize and filter.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
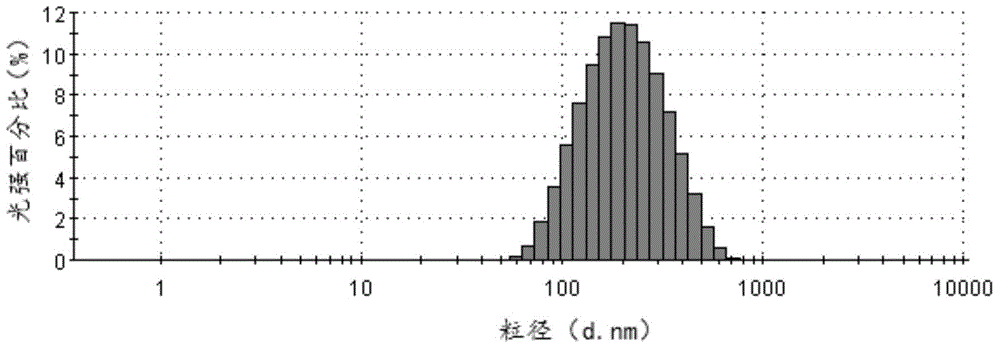
[0040]Add 300mg of cabazitaxel to a 50ml bottle, add 20ml of ethanol and chloroform mixed solvent (ethanol: chloroform volume ratio = 1:1), dissolve it and homogenize it under high-speed shear at 20,000 revolutions per minute (rpm). Disperse in 750ml, 1mg / ml albumin aqueous solution. Then transfer to a homogenizer, emulsify at 15,000 pounds per square inch (psi), and the resulting system is transferred to an ultrafiltration concentration device (PALL, 100KD membrane bag), and ultrafiltered to 50ml. The average particle diameter of the produced cabazitaxel composition particles is 180nm (MalvernNano-ZS90, see figure 1 ), the Zeta potential is -15.3mv, and the pH is 6.95. Sterilize by filtering through a 0.22 μm sterile filter head, and freeze-dry for 48 hours. Through HPLC analysis and detection, the drug loading capacity of the cabazitaxel albumin composition was calculated to be 25.4%, and the encapsulation efficiency was 93.2%.
Embodiment 2
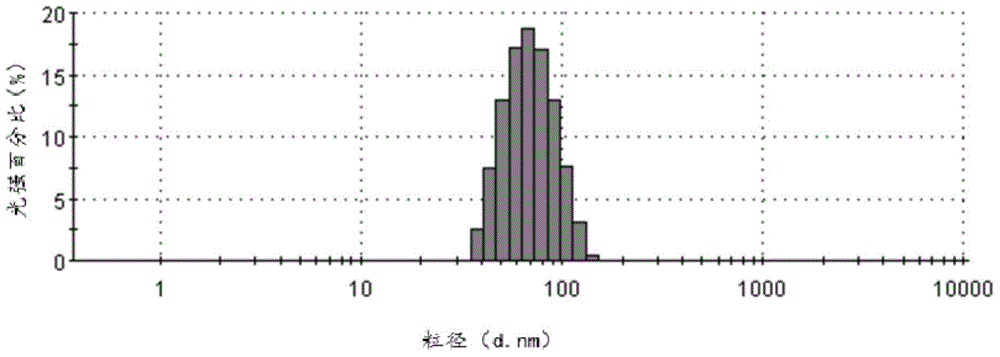
[0042] Add 200mg of cabazitaxel to a 50ml bottle, add 15ml of ethanol and chloroform mixed solvent (ethanol: chloroform volume ratio = 1:4), ultrasonically dissolve it and put it under high-speed shear at 30,000 revolutions per minute (rpm) Evenly dispersed in 50ml of saline containing 15mg / ml albumin. Then transfer to a homogenizer, emulsify at 20,000 pounds per square inch (psi), and the resulting system is transferred to an ultrafiltration concentration device (PALL, 100KD membrane bag), and ultrafiltered to 25ml. The average particle size of the produced cabazitaxel composition particles is 153nm (MalvernNano-ZS90), and the Zeta potential is -20.8mv (see Figure 4 ), the pH is 7.03. Sterilize by filtering through a 0.22 μm sterile filter head, and freeze-dry for 48 hours. Through HPLC analysis and detection, the calculated drug loading capacity of the cabazitaxel albumin composition is 18.2%, and the encapsulation efficiency is 95.7%.
Embodiment 3
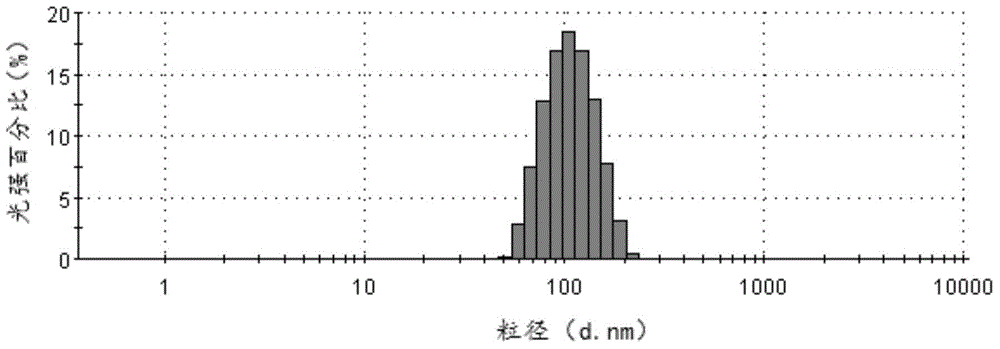
[0044] Add 150 mg of cabazitaxel to a 50 ml bottle, add 10 ml of acetone and chloroform mixed solvent (acetone: chloroform volume ratio = 1:5), ultrasonically dissolve it and put it under high-speed shear at 40,000 revolutions per minute (rpm) Evenly dispersed in 15ml of 5% glucose solution containing 50mg / ml albumin. Then transfer to a homogenizer, emulsify at 25,000 pounds per square inch (psi), and the resulting system is transferred to an ultrafiltration concentration device (PALL, 100KD membrane bag), and ultrafiltered to 8ml. The resulting cabazitaxel composition particles had an average particle size of 138 nm (MalvernNano-ZS90), a Zeta potential of -32.4 mv, and a pH of 7.03. Sterilize by filtering through a 0.22 μm sterile filter head, and freeze-dry for 48 hours. According to HPLC analysis and detection calculation, the drug loading capacity of the cabazitaxel albumin composition is 13.4%, and the encapsulation efficiency is 98.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric potential | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com