Pharmaceutical composition of ciclopirox olamine and medical application of pharmaceutical composition
A technology of ciclopirox amine and composition, which is applied in the directions of drug combination, metabolic diseases, urinary system diseases, etc., can solve the problems such as no reports related to ciclopirox amine cardiomyopathy, and achieves outstanding substantive characteristics and strong effect. , the effect of significant improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
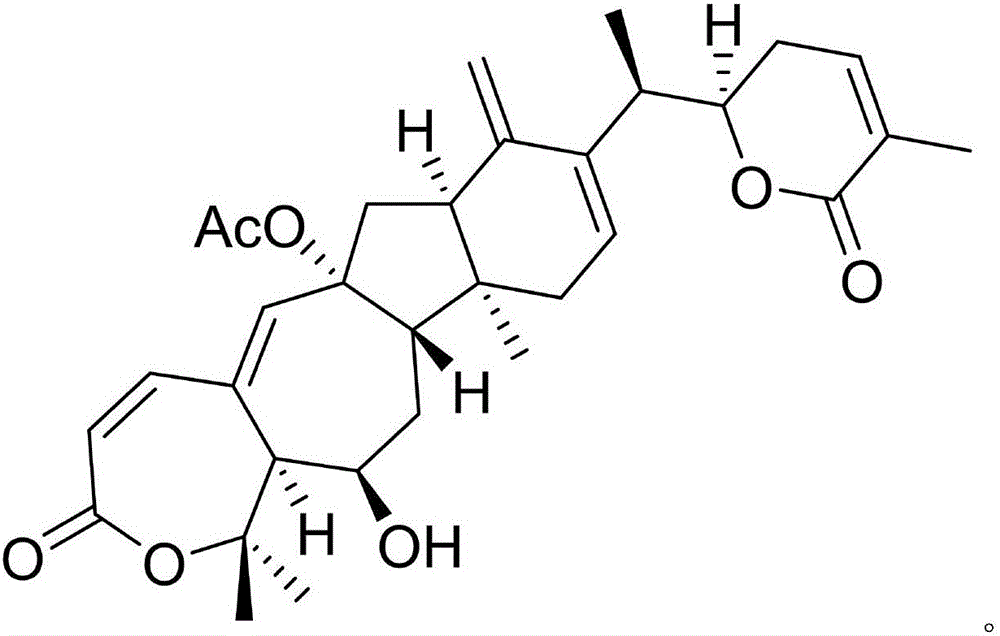
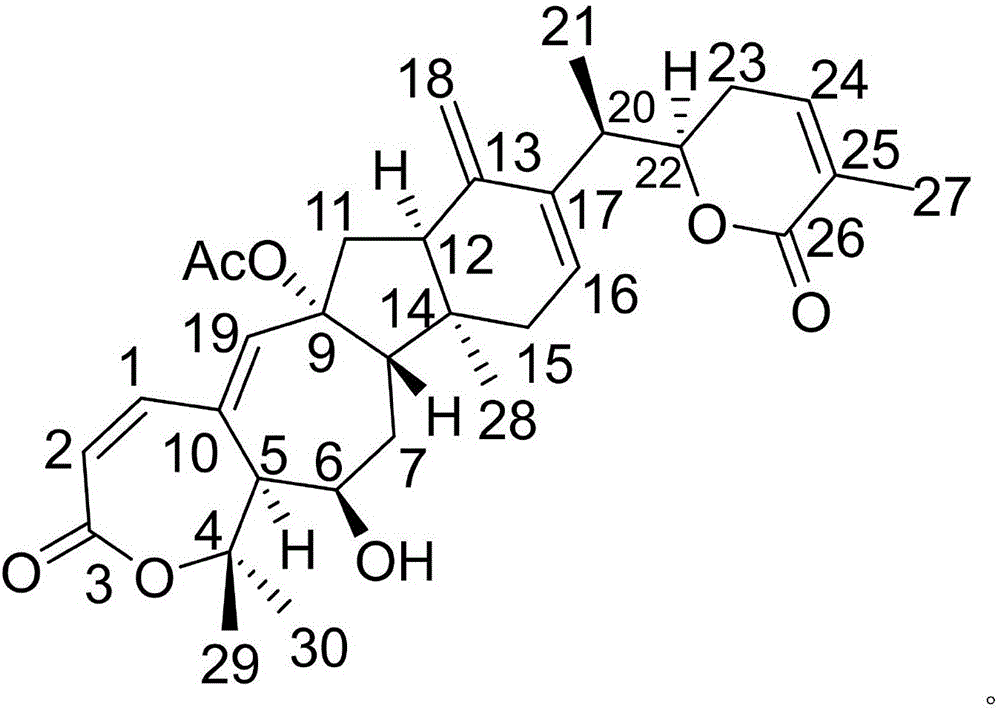
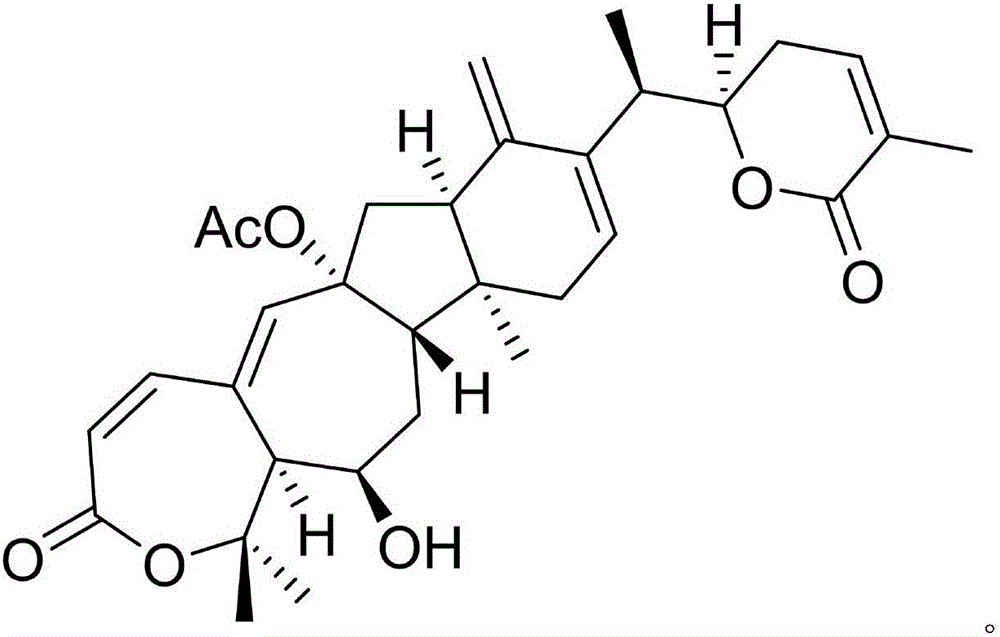
[0020] Example 1: Compound (I) Separation Preparation and Structure Confirmation
[0021] Sources of reagents: ethanol, petroleum ether, ethyl acetate, n-butanol, and dichloromethane were of analytical grade, purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Methanol, of analytical grade, were purchased from Jiangsu Hanbang Chemical Reagent Co., Ltd.
[0022] Separation method: (a) Crush asters (2kg), extract with 80% ethanol under heat reflux (15L×3 times), combine the extracts, concentrate until no alcohol smell (3L), and successively use petroleum ether (3L×3 times) , ethyl acetate (3L × 3 times) and water-saturated n-butanol (3L × 3 times) were extracted to obtain sherwood oil extract, ethyl acetate extract and n-butanol extract respectively; (b) step (a) Use D101 macroporous resin to remove impurities from the n-butanol extract, first elute with 10% ethanol for 8 column volumes, then use 70% ethanol for 10 column volumes, collect 70% eluate, and concentrate unde...
Embodiment 2
[0025] Embodiment 2: pharmacological action
[0026] 1. Materials and methods
[0027] 1.1 Animals
[0028] 40 Wistar rats aged 3 to 4 months, half male and half male, weighing 210 g to 250 g, were provided by the Experimental Animal Center of Jiamusi University. The animals were kept in a ventilated and dry environment with a temperature of (22±1)°C, a relative humidity of 55%-65%, and a 12-h light cycle, and were fed with maintenance diet 1022 for rats and mice.
[0029] 1.2 Reagents and samples
[0030] Ciclopirox was purchased from China National Institutes for Food and Drug Control. Compound (I) is self-made, and the preparation method is shown in Example 1. Streptozotocin (STZ) was purchased from Sigma Company of the United States; SOD, Coomassie Brilliant Blue protein assay kits were purchased from Nanjing Jiancheng Bioengineering Institute. Triglyceride kit was purchased from Changchun Huili Engineering Technology Development Center.
[0031] 1.3 Instruments
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com