Applications of CB2R (cannabinoid receptor 2) agonist in preparing medicines for treating hypertensive cerebral hemorrhage
A technology of hypertensive cerebral hemorrhage and cannabinoid receptors, applied in the field of preparation of drugs for the recovery of neurological function and cerebral edema after hypertensive cerebral hemorrhage, to achieve the effect of preventing brain atrophy, reducing the degree of edema, and increasing the transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
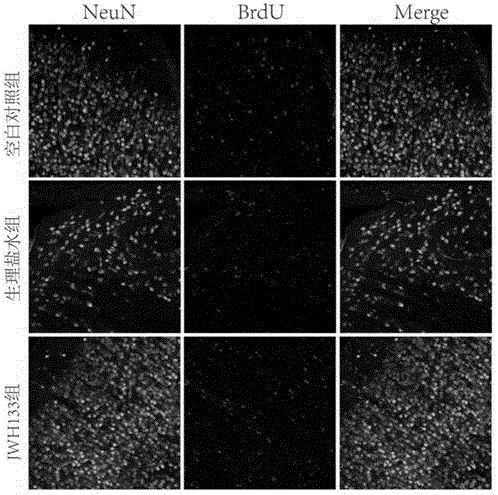
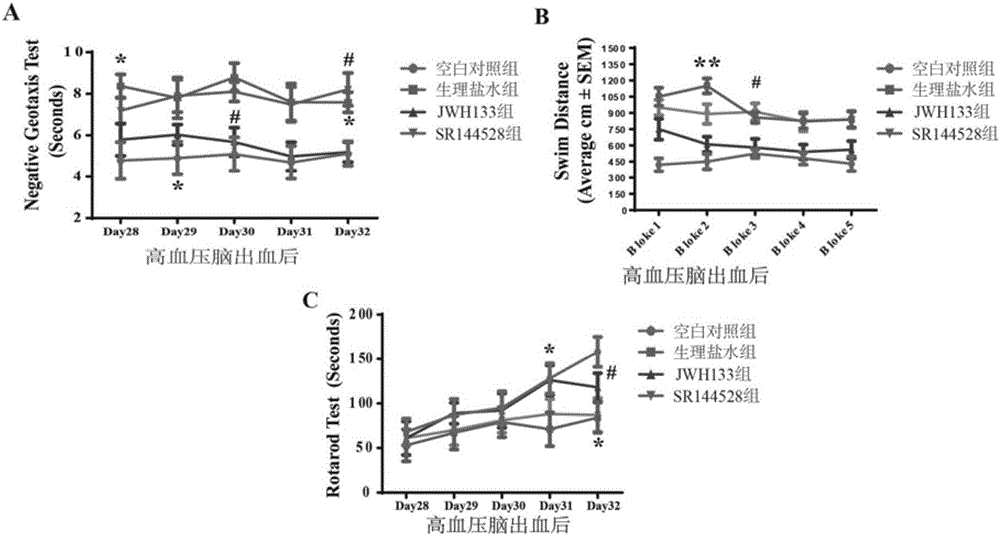
[0044] SD rats (250g-300g) were model animals, and the right femoral artery was dissected and separated, and 200 microliters of autologous arterial blood (without anticoagulant) was taken with a 1ml insulin needle and immediately injected into the right side of the rat through a stereotaxic instrument and a microinjection pump. In the basal ganglia area (slowly inject within 15 minutes), the needle insertion coordinates are 0.2 mm on the mouth side and 2.2 mm on the right side, and the needle tip is inserted 5 mm after breaking through the dura mater. Set up a blank control group (normal SD rats without surgery), a normal saline group (peritoneal injection of the same amount of normal saline after surgery), a JWH133 treatment group (peritoneal injection of JWH133 aqueous solution 1.5 mg / kg, twice a day, 7 days in total) and the type II cannabinoid receptor selective antagonist group (injection of SR144258), 10 animals in each group.
[0045]28 days after the model was establis...
Embodiment 2
[0053] Minocycline, also known as "minocycline" or "minocycline", is a broad-spectrum antibacterial tetracycline antibiotic with pharmacological properties such as long half-life and lipophilicity.
[0054] Due to the blood-brain barrier in the brain, direct administration of JWH133 is not easy to enter the brain to realize its function. After patients took it, it was found that the effect of JWH133 combined with minocycline was significantly better than that of patients who took JWH133 alone.
[0055] In view of this, the following animal experiments were carried out:
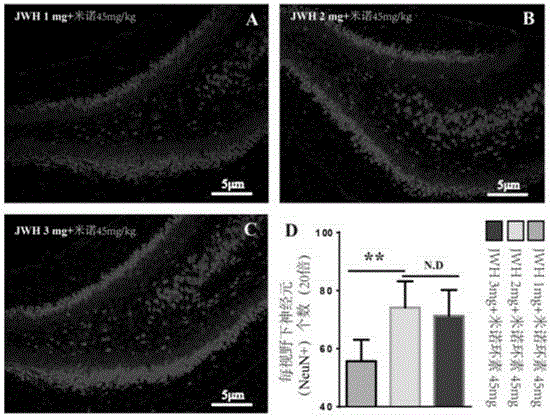
[0056] Experimental group 1: Type VII collagenase induced neonatal 7-day (P7) SD rat cerebral hemorrhage with intraventricular hemorrhage (GMH / IVH) model was the same as in Example 1, divided into sham operation group, normal saline group, JWH133 group alone and JWH133+ rice In the nocycline group, the brain water content of young mice was detected after 3 days of administration. The result is as Figure 4 ...
Embodiment 3
[0064] The medicine provided by the present invention includes type II cannabinoid receptor agonist and pharmaceutically acceptable auxiliary materials, and is made into injections, injections, tablets, granules, granules, pills, capsules, suspensions, emulsions, ointments, creams, Transdermal patches, etc.
[0065] The preparation of each dosage form is illustrated as follows:
[0066] 1. Preparation of injection
[0067] Weigh 1.5g type II cannabinoid receptor agonist, add 50g PEG-400, dissolve; prepare 50g pH5.5 phosphate buffer, weigh 0.02g sodium bisulfite and add to the above phosphate buffer; add the buffer Into the PEG solution, mix well, add 0.01% activated carbon for needles and keep warm at 100°C for 30 minutes, filter through a G3 vertically fused glass funnel, and filter through a 0.22 μm microporous membrane. The subpackaged injection can be directly pressed into a stopper or pressed into an aluminum cap to form an injection.
[0068] 2. Preparation of freeze-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com