A kind of preparation method of cbz-valganciclovir
A technology of ganciclovir and ganciclovir condensate is applied in the field of preparation of CBZ-valganciclovir, and achieves the effects of high production efficiency, reduced production cost and reduced environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
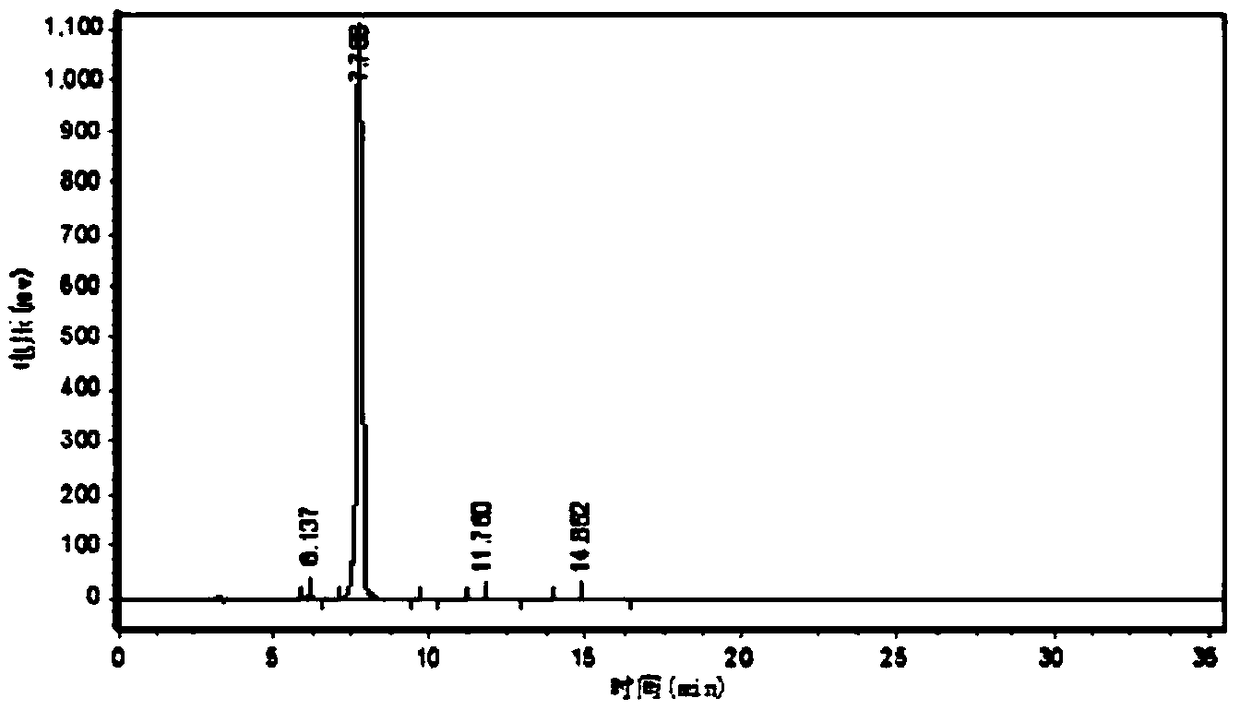
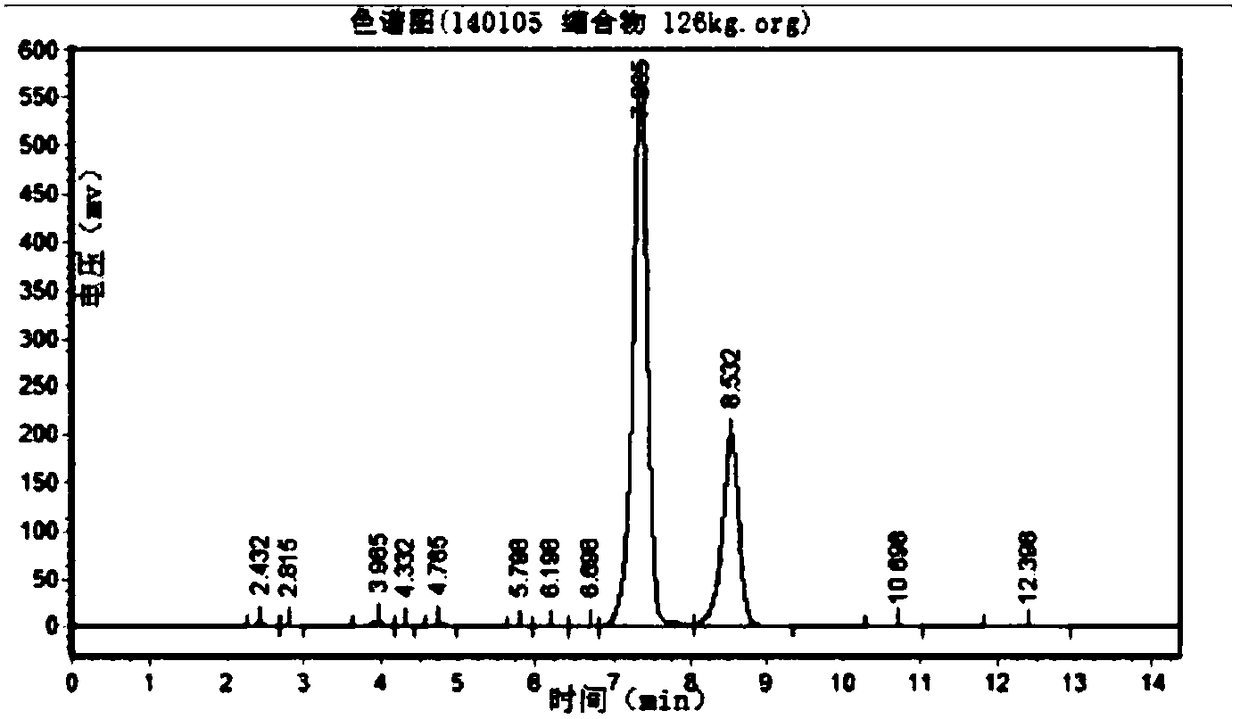
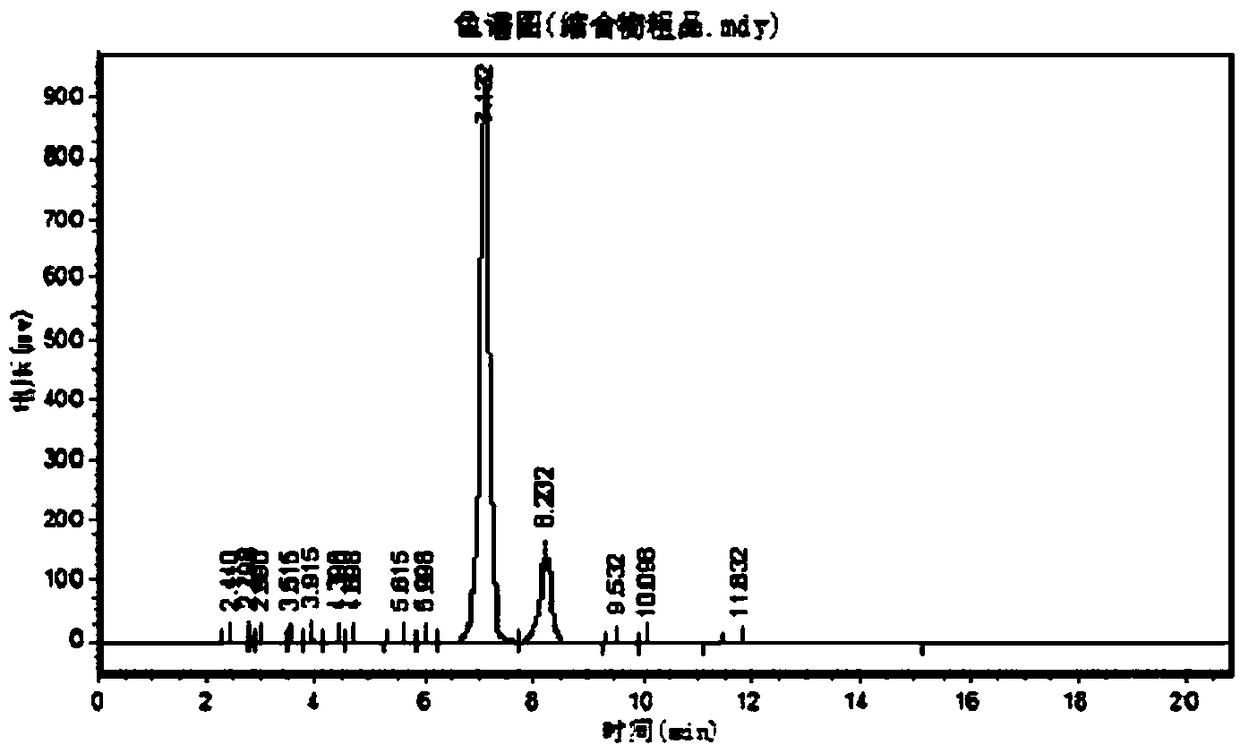
Image
Examples
Embodiment 1
[0080] A kind of CBZ-valganciclovir preparation method, it comprises the steps:
[0081] (1) 40kg ganciclovir condensate 7-position isomer, 3.0kg p-toluenesulfonic acid, 400kg solvent-DMF, 77kg1,3-diacetoxy-2-(acetoxymethoxy)propane, 60kg Put diacetylguanine into a 1000L reaction kettle, add 40kg of acetic anhydride, keep warm at 80-140°C for 3-5h and stir, then distill the acetic anhydride, continue to keep warm at 120-130°C for 18 hours, and distill 2- 3 times of acetic anhydride, so that the chemical balance reacts to the ganciclovir condensate;
[0082] (2) After the reaction in the step (1) ends, concentrate under reduced pressure and evaporate acetic anhydride and solvent-DMF until there is basically no evaporation to obtain a residue;
[0083] (3) Add 500L of ethyl acetate to the residue obtained in step (2), then heat up to reflux, cool down to 0-5°C, and keep warm for 1-24 hours for crystallization;
[0084](4) The product obtained in the step (3) is centrifuged and...
Embodiment 2
[0104] A kind of CBZ-valganciclovir preparation method, it comprises the steps:
[0105] (1) Put 100kg 7-position isomer of ganciclovir condensate, 3.0kg p-toluenesulfonic acid, 300kg solvent-DMF, 15kg1,3-diacetoxy-2-(acetoxymethoxy)propane into 1000L Add 100kg of acetic anhydride to the reaction kettle, keep it warm at 80-140°C for 3-5 hours and stir it, then distill the acetic anhydride, and continue to keep warm at 120-130°C for 18 hours, distilling the acetic anhydride 2-3 times during the period, so that Chemical equilibrium reaction to ganciclovir condensate;
[0106] (2) After the reaction in the step (1) ends, concentrate under reduced pressure and evaporate acetic anhydride and solvent-DMF until there is basically no evaporation to obtain a residue;
[0107] (3) Add 500L of ethyl acetate to the residue obtained in step (2), then heat up to reflux, cool down to 0-5°C, and keep warm for 1-24 hours for crystallization;
[0108] (4) The product obtained in the step (3) ...
Embodiment 3
[0118] A kind of CBZ-valganciclovir preparation method, it comprises the steps:
[0119] (1) 30kg ganciclovir condensate 7-isomer, 30kg ganciclovir 7-isomer, 2.3kg p-toluenesulfonic acid, 330kg solvent-DMF, 60kg1,3-diacetoxy-2- (Acetoxymethoxy) propane, 45kg diacetylguanine into a 1000L reactor, add 140kg acetic anhydride, keep warm at 80~140℃ for 3~5h and stir, then distill acetic anhydride, continue at 120-130 Keep warm for 18 hours under high temperature conditions, distill acetic anhydride 2-3 times during this period, so that the chemical equilibrium will react to the ganciclovir condensate;
[0120](2) After the reaction in the step (1) ends, concentrate under reduced pressure and evaporate acetic anhydride and solvent-DMF until there is basically no evaporation to obtain a residue;
[0121] (3) Add 500L of ethyl acetate to the residue obtained in step (2), then heat up to reflux, cool down to 0-5°C, and keep warm for 1-24 hours for crystallization;
[0122] (4) The pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com