Application of salvianolic acid A in independent-medicine or multiple-medicine-combined nephrotic syndrome treatment
A technology of nephrotic syndrome and salvianolic acid, applied in drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., to reduce the severity of kidney damage, reduce hormone resistance, and overcome side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 salvianolic acid A
[0049] 300 grams of salvianolic acid salt was diluted with water to a concentration of 1.0% (calculated as salvia magnesium acetate), adjusted to pH 6.0 with trisodium citrate, converted for 4 hours at 125°C and 0.15MPa, cooled to room temperature, and adjusted with hydrochloric acid. When the pH reaches 2.0-3.0, the content of salvianolic acid A is determined according to the law; the sample solution is passed through the HPD100 resin column, eluted with water and 25% ethanol solution (discarded), and then eluted with 40% ethanol solution to collect salvianolic acid A, according to the law Determine the content of salvianolic acid A, concentrate under reduced pressure until the concentration of salvianolic acid A is about 15.0 ~ 30.0mg / ml; the concentrated solution is passed through a CG161 resin column, eluted with 20% ethanol solution (discarded), and then washed with 35% ethanol solution Collect salvianolic acid A...
Embodiment 2
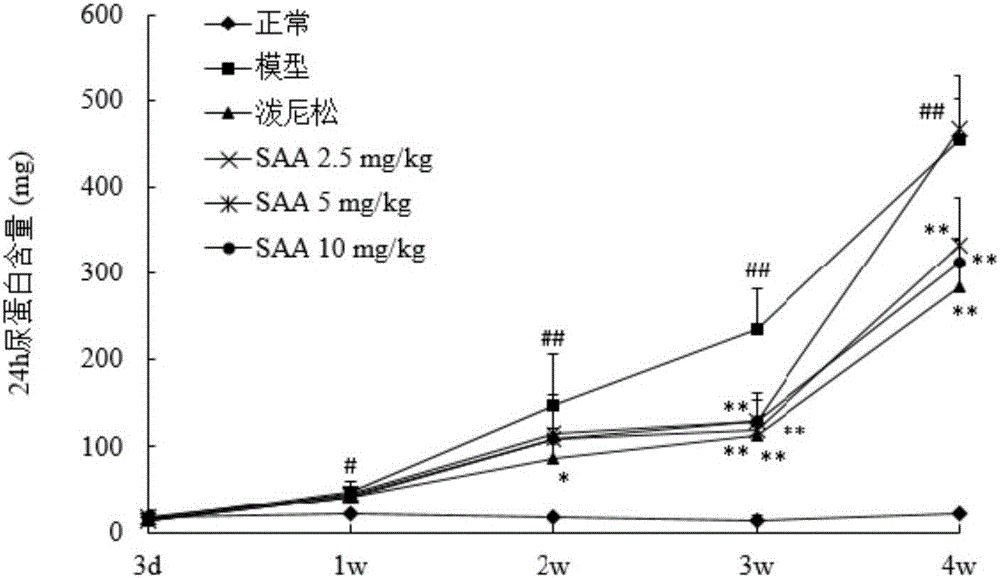
[0050] Example 2 The protective effect of salvianolic acid A on doxorubicin-induced nephrotic syndrome in rats
[0051] (1) Method
[0052] 1. Sample batch number and source
[0053] Salvianolic acid A, batch number 20130618 provided by Shandong Target Drug Research Co., Ltd., the preparation method is as in implementation 1.
[0054] Prednisone acetate tablets, batch number 130506, Zhejiang Xianju Pharmaceutical Co., Ltd.
[0055] Sodium chloride injection (0.9%), batch number A130529E1, Liaoning Minkang Pharmaceutical Co., Ltd.
[0056] 5% glucose, lot number B14012305, Shandong Hualu Pharmaceutical Co., Ltd.
[0057] 2. Model establishment and administration
[0058] Get 30 rats, be divided into 6 groups at random, be respectively: normal control group, model control group, positive drug group (prednisone acetate 10mg / kg, 0.5% CMC-Na suspension), salvianolic acid A ( 2.5, 5, 10 mg / kg, iv., in 5% glucose). Except for the normal control group, the rats in the other grou...
Embodiment 3
[0099] Example 3 The effect of salvianolic acid A on the nephropathy induced by puromycin aminonucleoside (PAN)
[0100] (1) Method
[0101] SD rats were taken, and a single tail vein injection of puromycin aminonucleoside (PAN) 15 mg / 100 g induced a rat nephropathy model. After PAN was injected for 7 days, the animals were administered in groups, respectively: normal control group, model control group, prednisone 10mg / kg (suspended with 0.5% CMC-Na, ig.), salvianolic acid A (5, 10mg / kg , dissolved in 5% glucose injection, iv.), salvianolic acid A5mg / kg+prednisone 10mg / kg, administered once a day, for 7 consecutive days, the normal control group and the model control group were given an equal volume of glucose solution. During the experiment, observe the general clinical signs of the animals, weigh the body weight, collect the 24h urine of the rats on the 3rd, 7th, and 14th day, centrifuge at 3000rpm for 15min, remove the sediment, collect the supernatant, and store it in a -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com